1-ethoxy-1,3,3,5,5-pentafluorocyclotriphosphazene CAS#: 33027-66-6; ChemWhat Code: 396203
Identification
| Product Name | 1-ethoxy-1,3,3,5,5-pentafluorocyclotriphosphazene |
| IUPAC Name | 2-ethoxy-2,4,4,6,6-pentafluoro-1,3,5-triaza-2λ5,4λ5,6λ5-triphosphacyclohexa-1,3,5-triene |
| Molecular Structure | 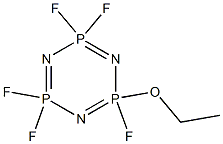 |
| CAS Registry Number | 33027-66-6 |
| MDL Number | MFCD28386107 |
| Synonyms | 1-ethoxy-1,3,3,5,5-pentafluorocyclotriphosphazene, ethoxy(pentafluoro)cyclotriphosphazene, pentafluoro ethoxy cyclotriphosphazene, pentafluoroethoxy cyclotriphosphazene, ethoxypentafluorocyclotriphosphazene, 2-ethoxy-2,4,4,6,6-pentafluoro-2λ5,4λ5,6λ5-cyclotriphosphazene, Aethyloxypentafluorcyclotriphosphazen |
| Molecular Formula | C2H5F5N3OP3 |
| Molecular Weight | 274.994 |
| InChI | InChI=1S/C2H5F5N3OP3/c1-2-11-14(7)9-12(3,4)8-13(5,6)10-14/h2H2,1H3 |
| InChI Key | CBTAIOOTRCAMBD-UHFFFAOYSA-N |
| Canonical SMILES | CCOP1(=NP(=NP(=N1)(F)F)(F)F)F |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2018/118768 | A Fluorination Method for Phosphonitrilic Chloride Trimer and Its Derivatives | 2018 |
| CN105636970 | Amino substituted phosphazene compound manufacturing method, the non-aqueous electrolytic solution for secondary battery and method of manufacturing the non-aqueous secondary battery manufacturing method (by machine translation) | 2016 |
Physical Data
| Appearance | Colorless transparent liquid |
| Boiling Point | 42 °C |
| Refractive index | 1.3610-1.3650 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) |
| Chemical shifts | 1H |
| Chemical shifts | 13C |
| Chemical shifts | 19F |
| Chemical shifts | 31C |
Route of Synthesis (ROS)

| Conditions | Yield |
| With n-butylammonium methanoate; sodium fluoride In hexane at 150℃; for 20h; Reagent/catalyst; Solvent; Temperature; Experimental Procedure Dissolve 27.5 g of crystals of ethoxypentachlorocyclotriphosphazene in 200 ml of n-hexane to form an ethoxy pentachloro-cyclophosphazene solution, and add 27 g of sodium fluoride as a fluorinating agent to the solution.2g of butylamine formate was added as a catalyst and reacted at 150°C for 20h.After filtration and distillation under reduced pressure, the phosphazene derivative ethoxypentafluorocyclotriphosphazene was obtained in a yield of 99.6percent. | 99.6% |
| With sodium fluoride In acetonitrile at 80℃; for 5h; Experimental Procedure To the above obtained ethoxypentachloro cyclotriphosphazene in 200g of acetonitrile, 210g NaF was added at constant stirring at a temperature of 80 ° C. The fluorination reaction lasted for 5 hours. The reaction was distilled to give ethoxypentafluoro cyclotriphosphazene (PNF2) 3. | |
| With sodium fluoride; 1-methyl-3-(prop-2-enyl)-1H-imidazol-3-ium tetrafluoroborate at 130℃; for 12h; Reagent/catalyst; Temperature; Experimental Procedure Take 0.1 mole ethoxy pentachloride cyclotriphosphazene and add it into a flask with 100 ml 1-allyl-3-methylimidazolium tetrafluoroborate ionic liquid and then add 1 mole sodium fluoride. Fluoridate 12 hours at 130° C. and then distill at 160° C. to get high-purity pentafluoro ethoxy cyclotriphosphazene. |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Danger |
| GHS Hazard Statements | H314: Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] H318: Causes serious eye damage [Danger Serious eye damage/eye irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 290621 |
| Storage | Under the room temperature and away from light |
| Market Price | USD 524/kg |
| Use Pattern |
| 1-ethoxy-1,3,3,5,5-pentafluorocyclotriphosphazene CAS#: 33027-66-6 as a highly effective fire retardant, can be used for lithium-ion battery electrolyte, with low viscosity and low effect to the performance of lithium battery electrolyte, achieving a good fire retardant effect by adding a small amount. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

