10-Hydroxy-2-decenoic acid CAS#: 14113-05-4; ChemWhat Code: 100995
Identification
| Product Name | 10-Hydroxy-2-decenoic acid |
| IUPAC Name | (E)-10-hydroxydec-2-enoic acid |
| Molecular Structure | |
| CAS Registry Number | 14113-05-4 |
| EINECS Number | 808-119-7 |
| MDL Number | MFCD00204506 |
| Synonyms | 14113-05-4 10-Hydroxy-2-decenoic acid 10-hydroxydec-2-enoic acid Royal jelly acid 765-01-5 (E)-10-hydroxydec-2-enoic acid (E)-10-Hydroxy-2-decenoic acid Queen Bee Acid trans-10-Hydroxy-2-decenoic acid 2-Decenoic acid, 10-hydroxy-, (2E)- 10-Hydroxydecenoic acid 10-Hydroxy-2-decylenic acid 10-hydroxy-trans-2-decenoic acid 10-hydroxy-2E-decenoic acid 10-HDA 76B519G7TJ (2E)-10-hydroxydec-2-enoic acid NSC-87516 Royaljellyacid UNII-76B519G7TJ MFCD00204506 NSC87516 NSC 87516 2-Decenoic acid, 10-hydroxy-, (E)- Queen Bee Acid;(E)-10-Hydroxy-2-decenoic acid SCHEMBL285440 10H2DA CHEBI:78668 10-Hydroxy-2(E)-decenoic acid QHBZHVUGQROELI-SOFGYWHQSA-N DTXSID601045504 trans-10-hydroxydec-2-enoic acid (E)-10-oxidanyldec-2-enoic acid AMY22616 HY-N1363 10-Hydroxy-2-Decenoic Acid ,(S) LMFA01050157 s3827 AKOS006282270 AKOS025310134 (2E)-10-Hydroxy-2-decenoic acid # CCG-266478 10-HYDROXYDECENOIC ACID [INCI] AC-15652 AC-34577 AS-13924 AS-58742 (E)-10-hydroxydec-2-enoic acid;10-HDA 10-HYDROXY-DEC-(E)-2-ENOIC ACID CS-0016772 H1337 N-(2,4,6-trichlorophenoxy)ethyl-N-propylamine O10366 EN300-1704333 A807734 A838726 A865474 J-007465 Q16908844 Z1198152019 |
| Molecular Formula | C10H18O3 |
| Molecular Weight | 186.25 |
| InChI | InChI=1S/C10H18O3/c11-9-7-5-3-1-2-4-6-8-10(12)13/h6,8,11H,1-5,7,9H2,(H,12,13)/b8-6+ |
| InChI Key | QHBZHVUGQROELI-SOFGYWHQSA-N |
| Isomeric SMILES | C(CCC/C=C/C(=O)O)CCCO |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN103787879 | A royal jelly acid derivative and its preparation method and application | 2016 |
| US2004/204596 | Method for the preparation of unsaturated hydroxy fatty acids and their esters, their use in pharmaceutical and/or cosmetic preparations | 2004 |
Physical Data
| Appearance | White powder |
| Melting Point | 70-75°C |
| Loss On Drying | ≤1.0% |
| Melting Point, °C | Solvent (Melting Point) |
| 64 – 65 | Petroleum ether, diethyl ether |
| 63 – 64 | diethyl ether, pentane |
| 65 – 66 | |
| 61.3 | |
| 62.5 – 63.5 | diethyl ether |
| 63 – 64 | CH2Cl2 |
| 63 – 65 | diethyl ether, hexane |
| Boiling Point, °C |
| 251 |
| 250 – 252 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d1 | |
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Chemical shifts | 13C | chloroform-d1 | 100 |
| Chemical shifts, Spectrum | 1H | chloroform-d1 |
| Description (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands | |
| Bands | neat (no solvent) |
| Bands | KBr |
| Description (UV/VIS Spectroscopy) |
| Absorption maxima |
Route of Synthesis (ROS)
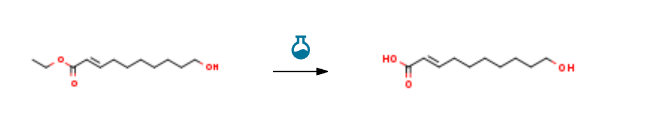
Route of Synthesis (ROS) of 10-Hydroxy-2-decenoic acid
| Conditions | Yield |
| With potassium hydroxide; water In ethanol for 8h; Experimental Procedure 119.6 g (0.56 mol) of hydroxyester was dissolved in 600 ml of ethanol and 400 ml of a 4.6 N solution of KOH was added. The medium was agitated for 8 h. The medium was extracted with isopropyl ether. The aqueous phase was acidified to pH=1 and extracted with ethyl acetate. After drying and evaporation, 99.6 g of pink solids were obtained. The solids were recrystallized in an isopropyl ether/petroleum ether mixture. The product was obtained in the form of a white solid (86 g, 83%). [0075] Characterization [0076] TLC: Rf=0.2 (heptane/ethyl acetate 7/3) [0077] Melting point: mp=61.3° C. [0078] 1H NMR (400 MHz, CDCl3): 7.06 (dt, 1H, J=15.6 and 7 Hz); 5.81 (dt, 1H, J=1.5 and 15.6 Hz); 3.64 (t, 2H, J=6.6 Hz); 2.22 (dq, 2H, J=1.2 and 7.3 Hz); 1.52-1.58 (m, 2H); 1.45-1.48 (m, 2H); 1.33-1.3765 (m, 6H). | 83% |
| With potassium hydroxide In ethanol for 1.5h; Heating; | 73% |
| With sodium hydroxide In ethanol for 5h; Heating; | 62% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] |
| Precautionary Statement Codes | P264, P264+P265, P280, P302+P352, P305+P351+P338, P321, P332+P317, P337+P317, and P362+P364 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Store at room temperature for long time, in container tightly sealed; Protect from light. | |
| HS Code | |
| Storage | Store at room temperature for long time, in container tightly sealed; Protect from light. |
| Shelf Life | 3 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 186.251 |
| logP | 2.28 |
| HBA | 3 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 57.53 |
| Rotatable Bond (RotB) | 8 |
| Matching Veber Rules | 2 |
| Quantitative Results | ||
| 1 of 58 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | ||
| 2 of 58 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | LA REACTION DE WITTIG-HORNER EN MILIEU HETEROGENE VI. SELECTIVITE DE LA REACTION SUR DES COMPOSES BIFONCTIONNELS | |
| 3 of 58 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | The Physiology of Caste Development in Social Insects | |
| 4 of 58 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Royalactin extends lifespan of Caenorhabditis elegans through epidermal growth factor signaling | |
| 5 of 58 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | The influence of royal jelly and human interferon-alpha (HuIFN-αN3) on proliferation, glutathione level and lipid peroxidation in human colorectal adenocarcinoma cells in vitro |
| Use Pattern |
| 10-Hydroxy-2-decenoic acid possesses antioxidant properties, helping to combat the damage caused by free radicals and maintaining cellular health. And It is believed to have anti-inflammatory characteristics and can be used to alleviate inflammation-related health issues. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |