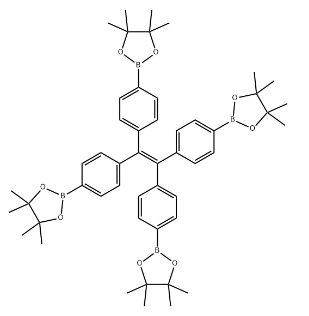
1,1,2,2-Tetrakis(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)ethene CAS#: 1660996-72-4; ChemWhat Code: 1491394
Identification
Physical Data
No data available
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 24.84 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | ||
| Chemical shifts | 1H | chloroform-d1 | 400 | |
| Chemical shifts | 1H | chloroform-d1 | 300 | |
| Chemical shifts | 1H | chloroform-d1 | 400 | |
| Chemical shifts | 1H | chloroform-d1 | ||
| Chemical shifts | 13C | chloroform-d1 |
| 1,1,2,2-Tetrakis(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)ethene CAS#: 1660996-72-4 NMR | 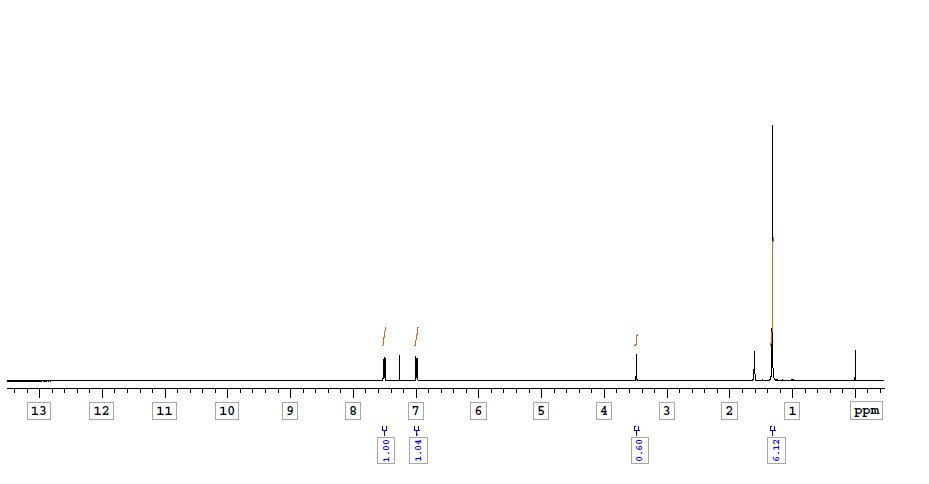 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | potassium bromide |
| Bands, Spectrum |
Route of Synthesis (ROS)

| Conditions | Yield |
| Stage #1: 1,1,2,2-tetrakis(4-bromophenyl)ethene; bis(pinacol)diborane With potassium acetate In 1,4-dioxane for 1h; Inert atmosphere; Stage #2: With (1,1′-bis(diphenylphosphino)ferrocene)palladium(II) dichloride In 1,4-dioxane Inert atmosphere; Experimental Procedure 5.1 (1) Preparation of tetrakis (pinacol borate) tetraphenylene Put tetrabromotetraphenylethylene (0.1mmol), bipinacol borate (1.0mmol), potassium acetate (1mmol) into the 1,4-dioxane solvent single-neck bottle equipped with 30mL, pass nitrogen 60 minute. Weigh 1,1′-bisdiphenylphosphinoferrocene palladium dichloride, put it into the above-mentioned single-necked flask, and let it pass nitrogen for half an hour. Continue heating to reflux in a nitrogen atmosphere overnight, cool the reaction system, pour the reaction solution into a beaker containing 500 mL of water, and extract with dichloromethane. The organic phase was collected and dried, mixed with silica gel, and sampled by dry method, and purified by column using dichloromethane/methanol (volume ratio of 10:1) as a developing solvent to obtain the intermediate tetrakis (pinacol borate) tetraphenyl Ethylene, 85% yield. | 85% |
| With (1,1′-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate In 1,4-dioxane Inert atmosphere; Reflux; Experimental Procedure 5.1 (1) Preparation of tetrakis (pinacol borate) tetraphenylene Put tetrabromotetraphenylethylene (0.1mmol), bipinacol borate (1.0mmol), potassium acetate (1mmol) into the 1,4-dioxane solvent single-neck bottle equipped with 30mL, pass nitrogen 60 minute. Weigh 1,1′-bisdiphenylphosphinoferrocene palladium dichloride, put it into the above-mentioned single-necked flask, and let it pass nitrogen for half an hour. Continue heating to reflux in a nitrogen atmosphere overnight, cool the reaction system, pour the reaction solution into a beaker containing 500 mL of water, and extract with dichloromethane. The organic phase was collected and dried, mixed with silica gel, and sampled by dry method, and purified by column using dichloromethane/methanol (volume ratio of 10:1) as a developing solvent to obtain the intermediate tetrakis (pinacol borate) tetraphenyl Ethylene, 85% yield. | 85% |
| With dichloro(1,1′-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate In 1,4-dioxane at 110℃; for 72h; Inert atmosphere; | 83% |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Other Data
| Transportation | Under the room temperature and away from light |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 836.297 |
| logP | -14.8 |
| HBA | 8 |
| HBD | 0 |
| Matching Lipinski Rules | 2 |
| Veber rules component | |
| Polar Surface Area (PSA) | 73.84 |
| Rotatable Bond (RotB) | 8 |
| Matching Veber Rules | 2 |
| Use Pattern |
| 1,1,2,2-Tetrakis(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)ethene has important applications in the field of materials science. It serves as a starting material for the synthesis of organic semiconductor materials, fluorescent materials, and light-emitting diode materials. By modifying and functionalizing its structure, the optical, electronic, and conductivity properties of materials can be controlled, enabling their use in optoelectronic devices, light sensors, organic solar cells, and other fields. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | https://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |