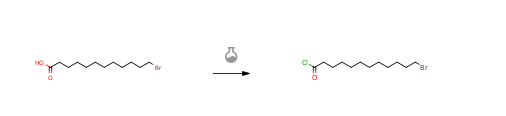
12-Bromododecanoic acid CAS#: 73367-80-3; ChemWhat Code: 1119834
Identification
| Product Name | 12-Bromododecanoic acid |
| IUPAC Name | 12-bromododecanoic acid |
| Molecular Structure | |
| CAS Registry Number | 73367-80-3 |
| EINECS Number | 277-401-4 |
| MDL Number | MFCD00002738 |
| Beilstein Registry Number | 1771588 |
| Synonyms | 12-Bromdodecansäure 12-Bromododecanoic acid 12-Bromolauric acid Acide 12-bromododécanoïque Dodecanoic acid, 12-bromo- 12-Brom-dodecansaeure 12-bromo dodecanoic acid 12-bromo-1-dodecanoic acid 12-bromo-dodecanoic acid 12-Bromododecanoicacid |
| Molecular Formula | C12H23BrO2 |
| Molecular Weight | 279.218 |
| InChI | InChI=1S/C12H23BrO2/c13-11-9-7-5-3-1-2-4-6-8-10-12(14)15/h1-11H2,(H,14,15) |
| InChI Key | YYKBWYBUCFHYPR-UHFFFAOYSA-N |
| Canonical SMILES | O=C(O)CCCCCCCCCCCBr |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN117624240 | Arctigenin mitochondrial targeting triphenylphosphine derivative, preparation method and application thereof, and drug delivery system | 2024 |
| US2013/131354 | Naphthocyanines for Use as Contrast Agents | 2013 |
| US2015/18515 | METHOD FOR PRODUCING EPOXY COMPOUND AND CATALYST COMPOSITION FOR EPOXIDATION REACTION | 2015 |
Physical Data
| Appearance | Off-white powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C | Solvent (Melting Point) |
| 54.9 – 55.3 | |
| 53 – 55 | |
| 49 – 50 | |
| 52 | hexane |
| Description (Adsorption (MCS)) | Partner (Adsorption (MCS)) |
| Adsorption | graphite |
| Further physical properties of the adsorbed molecule | MoS2 |
| Further physical properties of the adsorbed molecule | graphite |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 300 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 |
| 12-Bromododecanoic acid CAS#: 73367-80-3 NMR | 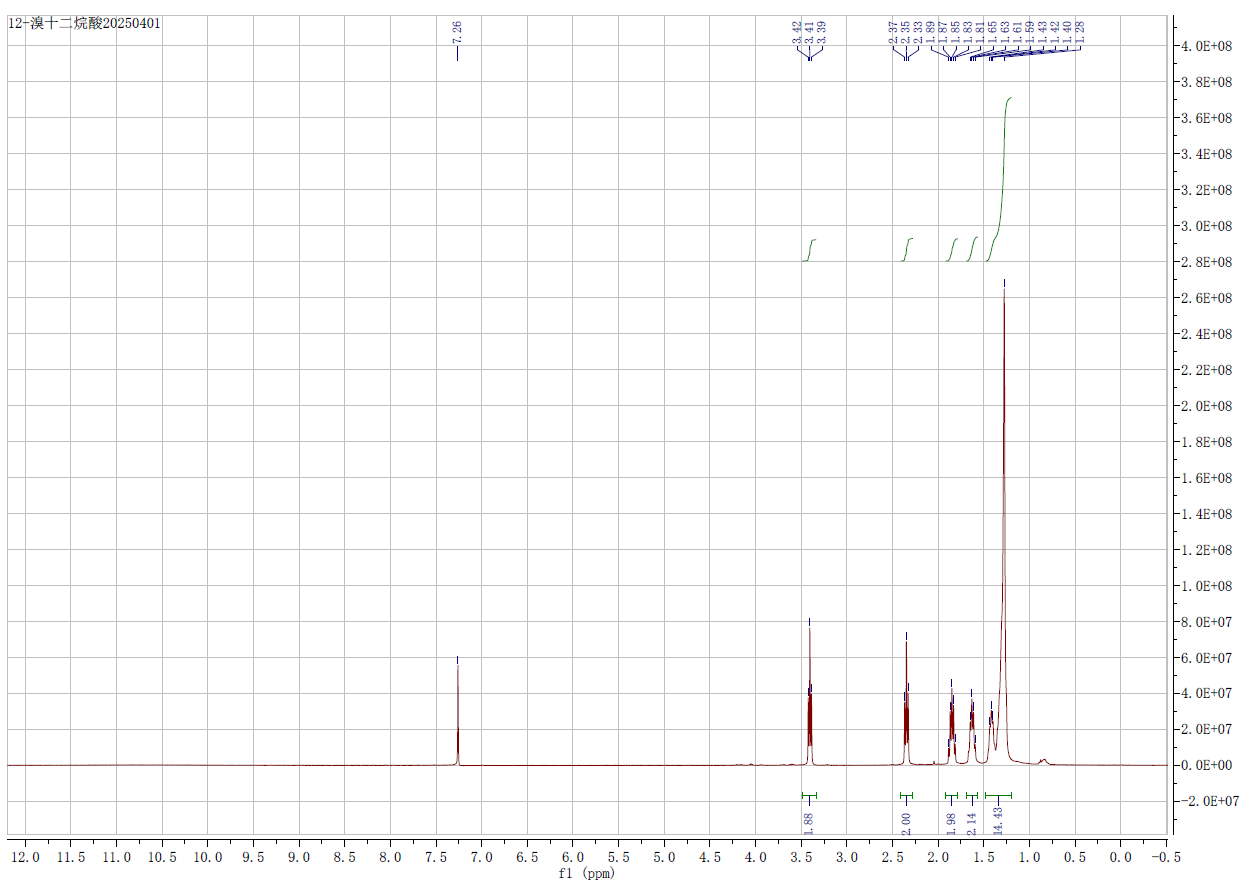 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | neat (no solvent, solid phase) |
| Description (Mass Spectrometry) |
| electron impact (EI), spectrum |
| high resolution mass spectrometry (HRMS), electrospray ionisation (ESI), liquid chromatography mass spectrometry (LCMS), time-of-flight mass spectra (TOFMS), spectrum |
Route of Synthesis (ROS)
| Conditions | Yield |
| With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 20℃; for 16h; | 100% |
| With oxalyl dichloride; N,N-dimethyl-formamide In chloroform at 45℃; for 2h; Cooling with ice; | 98% |
| With thionyl chloride In N,N-dimethyl-formamide at 80℃; for 1h; |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (66.7%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (66.7%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (66.7%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 279.217 |
| logP | 5.341 |
| HBA | 2 |
| HBD | 1 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 37.3 |
| Rotatable Bond (RotB) | 11 |
| Matching Veber Rules | 1 |
| Use Pattern |
| Organic synthesis intermediates |
| It is a very useful building block that can introduce esters and amides at the carboxylic acid position and perform nucleophilic substitution (SN2) reactions at the bromine position to construct more complex molecular structures. It is widely used in the development of surfactants, functional materials, pharmaceutical intermediates, etc. |
| Polymer modifiers / grafting initiators |
| It can be used to synthesize polymers with specific terminal functional groups, such as controlling polymerization reactions by bromine-initiated atom transfer radical polymerization (ATRP)**. The carboxylic acid part can be used to graft to the polymer backbone or further react to form ester and amide bonds. |
| Drug and bioactive molecule precursors |
| Although it is not often used as an active drug, it is also used in the construction of precursors in pharmaceutical chemistry or as part of a carrier because it has a long-chain alkyl structure and functional groups. |
| Functionalized material synthesis |
| In the synthesis of self-assembling materials containing bromine functional groups (such as amphiphilic molecules, surfactants, liquid crystal monomers), it is used as one of the building blocks. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |