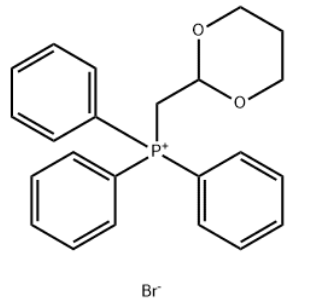
((1,3-dioxan-2-yl)methyl)triphenylphosphonium bromide CAS#: 73022-37-4; ChemWhat Code: 1489774
Identification
Physical Data
| Appearance | Powder |
Spectra
No data available
Route of Synthesis (ROS)
| Conditions | Yield |
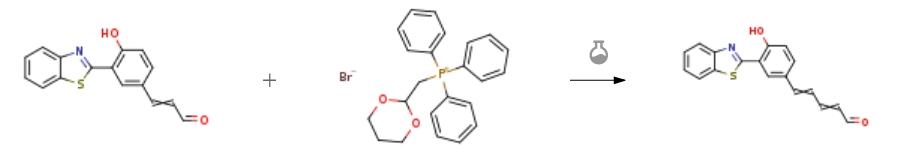
| With 18-crown-6 ether; sodium hydride In tetrahydrofuran at 0 – 60℃; for 0.166667h; Wittig-Horner Reaction; Experimental Procedure 9.1 (1) Synthesis of compound KSLOH09 Compound KSLOH08 (2 g, 7.11 mmol) was dissolved in 50 mL of tetrahydrofuran,(1,3-dioxane-2-methyl)triphenylphosphonium bromide (6.104 g, 14.228 mmol) was added under stirring at 0°C,Sodium hydride (60% oil dispersion) (1.422 g, 35.55 mmol),18-crown-6 ether (178mg, 25mg/mmol),After stirring for 10 min,The temperature was raised to 50-60°C, and the reaction was carried out overnight.The next day, a small amount of water was added to the reaction solution to quench,Then 1M HCl solution was added to the reaction solution and stirred.TLC monitoring,After the reaction is over,Adjust pH to 7 with ammonia,The mixture was extracted three times with ethyl acetate,Combine the organic phases,After drying with anhydrous Na2SO4,spin dry,A total of 2 g of KSLOH09 were obtained by column chromatography,as a yellow solid,Yield was 92%. | 92% |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Other Data
| Transportation | Store at 2~8° for long time. |
| HS Code | |
| Storage | Store at 2~8° for long time. |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 443.32 |
| logP | 7.198 |
| HBA | 2 |
| HBD | 0 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 18.46 |
| Rotatable Bond (RotB) | 5 |
| Matching Veber Rules | 2 |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |