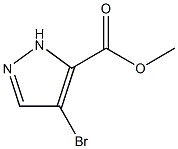
1H-Pyrazole-3-carboxylic acid, 4-bromo-, methyl ester CAS#: 81190-89-8; ChemWhat Code: 1188593
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2013/165464 | HETEROARYLS AND USES THEREOF | 2013 |
Physical Data
| Appearance | White powder |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) |
| Chemical shifts, Spectrum | 13C | chloroform-d1 |
Route of Synthesis (ROS)
| Conditions | Yield |
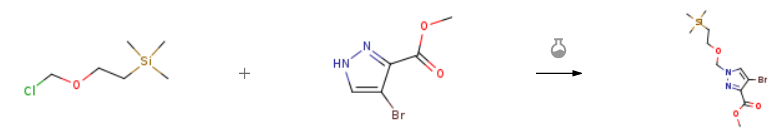
| Stage #1: methyl 4-bromo-1H-pyrazole-3-carboxylate With sodium hydride In tetrahydrofuran at 0℃; for 0.166667h; Stage #2: (2-trimethylethylsilylethoxy)methyl chloride In tetrahydrofuran at 20℃; Experimental Procedure Intermediate 4A. Methyl 4-bromo-1-((2-(trimethylsiliyl)ethoxy)methyl)-1H-pyrazole-3carboxylate To a suspension of methyl 4-brorno-1H-pyrazole-3-carboxylate (2.45 g, 11.9 rnmol) in THF (120 rnL) at () °C, was added 60% Nal-I (0.621 g, 15.5 mrnol) portionwise.The reaction was stirred at 0 °C for 10 mm, then 2-(trirnethylsilyi)ethoxymethyl chloride (254 mL, 14.34 mrnoi) was added dropwise. The suspension was warmed slowly to rt overnight. Then, it was cooled down to 0 °C, MeOH was added and the solvents were removed in vacuo. The residue was diluted with EtOAc and sat. Nal-ICOs. The aqqueous layer was extracted 2x with EtOAc. The organic layer was washed with brine, dried overMgSO4 and concentrated. Purification by flash chromatography (80 g column, 0-50% E:tOAc in Hexanes) afforded intermediate 4A as a colorless oil (3.17 g, 87%). ‘H NMR (400 MHz. CFILOROFORM-d) d 7.73 (s, IH), 5.48 (s, 2H), 3.97 (s, 3H), 3.63 – 3.56 (iii. 2H). 093 (t, .J=8.3 Hz, 2H). 000 (s. 9H). LCMS [M + H] = 337.0, | 87% |
| Stage #1: methyl 4-bromo-1H-pyrazole-3-carboxylate With sodium hydride In tetrahydrofuran at 0℃; for 0.5h; Stage #2: (2-trimethylethylsilylethoxy)methyl chloride In tetrahydrofuran at 0℃; for 5h; Experimental Procedure To a solution of methyl 4-bromo-1H-pyrazole-3-carboxylate (10.0 g, 48.8 mmol) in THF (150.00 mL) was added NaH (2.341 g, 58.5 mmol) portion wise at 0 °C. The reaction mixture was stirred for 30 min, then SEM-Cl (10.38 mL, 58.5 mmol) was added. The reaction mixture was stirred at 0 °C for 5 h. The reaction was quenched with water (30 mL). The reaction mixture separated into two layers, and the aqueous layer was extracted with EtOAc (2 X 50 mL). The combined organic extracts were dried (Na2SO4) and concentrated to yield crude compound. The crude compound was purified by ISCO using 120 g silica column, the compound was eluted in 20% EA in hexanes, the fractions were collected and concentrated to afford methyl 4-bromo-1-((2- (trimethylsilyl)ethoxy)methyl)-1H-pyrazole-3-carboxylate (13.95 g, 41.6 mmol, 85 % yield) as an oil. LCMS Retention time: 1.63 min [A], MS (E+) m/z: 337.1 [M+2H]. | 85% |
| Stage #1: methyl 4-bromo-1H-pyrazole-3-carboxylate With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 0.5h; Inert atmosphere; Stage #2: (2-trimethylethylsilylethoxy)methyl chloride In tetrahydrofuran; mineral oil at 20℃; Experimental Procedure 3 Method C3: SEM Protection of Pyrazoles Illustrative Example 4-Bromo-1-(2-trimethylsilanyl-ethoxymethyl)-1H-pyrazole-3-carboxylic acid methyl ester General procedure: The pyrazole (1 eq) is dissolved in dry THF at 0° C. After addition of NaH (1.5 eq), the mixture is stirred at 0° C. under N2 atmosphere for 30 min. Next, SEM-C1 (1.5 eq) is slowly added and the resulting mixture is stirred at room temperature overnight. Subsequently, the mixture is quenched with water and extracted with EtOAc. The organic phase is isolated, dried over Na2SO4, filtered and evaporated to give a residue that is used as such. The pyrazole (Int 4, 2 g, 9.76 mmol) is dissolved in dry THF (20 mL) at 0° C. After addition of NaH (60 w %, 584 mg, 14.6 mmol), the mixture is stirred at 0° C. under N2 atmosphere for 30 min. Next, SEM-C1 (2.58 mL, 14.6 mmol) is slowly added and the resulting mixture is stirred at room temperature overnight. Subsequently, the mixture is quenched with water and extracted with EtOAc. The organic phase is isolated, dried over Na2SO4, filtered and evaporated to give a residue that is used as such. |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H302 (66.7%): Harmful if swallowed [Warning Acute toxicity, oral] H312 (33.3%): Harmful in contact with skin [Warning Acute toxicity, dermal] H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H332 (66.7%): Harmful if inhaled [Warning Acute toxicity, inhalation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P264, P264+P265, P270, P271, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P317, P319, P321, P330, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 |
Other Data
| Transportation | Under the room temperature and away from light |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 205.011 |
| logP | 1.828 |
| HBA | 4 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 54.98 |
| Rotatable Bond (RotB) | 2 |
| Matching Veber Rules | 2 |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |