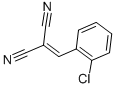
2-(2-Chlorobenzylidene)malononitrile CAS#: 2698-41-1; ChemWhat Code: 61728
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN108383755 | Synthesis method of alkenyl dinitrile compounds | 2018 |
| CN104497007 | A chiral indole and ABS synthetic method of the compound | 2017 |
| US4140710 | Recovery of o-chlorobenzilidene malononitrile from finely divided mixtures thereof with colloidal silica | 1979 |
| US4418210 | Process for producing asymmetrical thioureas | 1983 |
Physical Data
| Melting Point, °C |
| 93 – 95 |
| 94 – 96 |
| 96 – 98 |
| 90 – 92 |
| 85 – 87 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.389 | 24.85 |
| Description (Association (MCS)) | Partner (Association (MCS)) |
| Adsorption | pyrographite |
| Rate of adsorption | pyrographite |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d1 | |
| Chemical shifts | 13C | chloroform-d1 | |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | |
| Chemical shifts, Spectrum | 13C | dimethylsulfoxide-d6 | |
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | potassium bromide |
| Bands, Spectrum | |
| Bands | |
| Bands | KBr |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| methanol | 287 | ||
| Absorption maxima |
Route of Synthesis (ROS)
| Conditions | Yield |
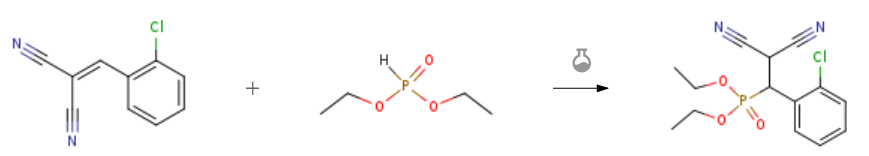
| With mercapto-functionalized silica-coated nano γ-Fe2O3-pyridine In neat (no solvent) at 70℃; for 1.5h; Michael Addition; Green chemistry; Experimental Procedure 2.3. General procedure for Michael addition of diethyl phosphiteto ,α,β-unsaturated malonates catalyzed by nano-Fe2O3-pyridine based catalyst 1 General procedure: Catalyst 1 (5 mol%) was added to a mixture of ,-unsaturatedmalonate (5 mmol) and diethyl phosphite (10 mmol). The mixturewas stirred at 70 C for the appropriate time (Table 2). The catalystwas separated by a magnetic bar from the cooled mixture,washed with EtOH, dried 30 min at 110 C and reused for a consecutiverun under the same reaction conditions. Evaporation of thesolvent of the remaining solution under reduced pressure gave thecrude products. The pure products (2-18, Table 2) were isolated bychromatography on silica gel eluted with n-hexane:EtOAc (1:2). | 95% |
| With 3-aminopropylated silica gel at 50℃; for 0.25h; phospha-Michael addition; Neat (no solvent); | 91% |
| With iron-doped single walled carbon nanotubes In neat (no solvent) at 50℃; for 3h; Green chemistry; chemoselective reaction; Experimental Procedure 4.3.2 Solvent-free synthesis of β-phosphonomalonates catalyzed by Fe/SWCNTs General procedure: The required malonates (1 mmol) and phosphorus compound (1.2 mmol) were added to Fe/SWCNTs (0.05 g) and the mixture was heated in an oil bath at 50 °C. After the reaction was complete, EtOAc (4×10 mL) was added to the reaction mixture and centrifuged to separate the catalyst. The organic solvent was removed under reduced pressure. After purification by plate chromatography (solvent: n-hexane/ethyl acetate, 50:50) the product was obtained. | 90% |
Safety and Hazards
| Pictogram(s) |     |
| Signal | Danger |
| GHS Hazard Statements | H301 (98.44%): Toxic if swallowed [Danger Acute toxicity, oral] H315 (31.25%): Causes skin irritation [Warning Skin corrosion/irritation] H317 (92.19%): May cause an allergic skin reaction [Warning Sensitization, Skin] H319 (31.25%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H330 (29.69%): Fatal if inhaled [Danger Acute toxicity, inhalation] H334 (62.5%): May cause allergy or asthma symptoms or breathing difficulties if inhaled [Danger Sensitization, respiratory] H335 (32.81%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] H400 (90.62%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H411 (29.69%): Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] |
| Precautionary Statement Codes | P233, P260, P261, P264, P264+P265, P270, P271, P272, P273, P280, P284, P301+P316, P302+P352, P304+P340, P305+P351+P338, P316, P319, P320, P321, P330, P332+P317, P333+P317, P337+P317, P342+P316, P362+P364, P391, P403, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Under the room temperature and away from light | |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 188.616 |
| logP | 2.28 |
| HBA | 2 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 47.58 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Use Pattern |
| 2-(2-Chlorobenzylidene)malononitrile CAS#: 2698-41-1 has extensive applications in exploring the effects of irritants on the human body, and is used as tear gas and riot control agents. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Limited | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |