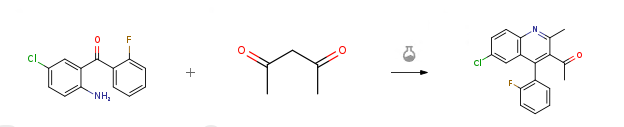
2-Amino-5-chloro-2′-fluorobenzophenone CAS#: 784-38-3; ChemWhat Code: 1007192
Identification
| Product Name | 2-Amino-5-chloro-2′-fluorobenzophenone |
| IUPAC Name | (2-amino-5-chlorophenyl)-(2-fluorophenyl)methanone |
| Molecular Structure | 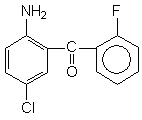 |
| CAS Registry Number | 784-38-3 |
| EINECS Number | 212-316-8 |
| MDL Number | MFCD00038381 |
| Beilstein Registry Number | No data available |
| Synonyms | 2-amino-5-chloro-2′-fluorobenzophenone2-amino-5-chlorophenyl-2-fluorophenylmethanone2-Amino-5-chlor-2′-fluorbenzophenon2-amino-5-chloro-2′-fluorobenzophenone2-amino-5-chloro-2’-fluorobenzophenone |
| Molecular Formula | C13H9ClFNO |
| Molecular Weight | 249.669 |
| InChI | InChI=1S/C13H9ClFNO/c14-8-5-6-12(16)10(7-8)13(17)9-3-1-2-4-11(9)15/h1-7H,16H2 |
| InChI Key | GTGMXPIQRQSORU-UHFFFAOYSA-N |
| Canonical SMILES | Nc1ccc(Cl)cc1C(=O)c1ccccc1F |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN117229206 | Preparation method for synthesizing polysubstituted 2-quinolinone compound through base catalysis | 2023 |
| CN104447687 | A nitrogen-containing seven-membered ring derivatives of industrial production method | 2016 |
Physical Data
| Appearance | Yellow powder crystals |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C | Solvent (Melting Point) |
| 96 – 97 | |
| 63 – 65 | |
| 94 – 95 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 225 – 230 | 55 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Stability constant of the complex with … | H2O | 20 | Th(4+) |
| Association with compound | |||
| Enthalpy of association |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 125 |
| Chemical shifts | 1H | chloroform-d1 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | potassium bromide |
| Mid IR (MIR), Bands | nujol |
| Bands | potassium bromide |
| Description (Mass Spectrometry) |
| EI (Electron impact), Spectrum |
| FAB (Fast atom bombardment), Spectrum |
| ESI (Electrospray ionisation), HRMS (High resolution mass spectrometry) |
| FAB (Fast atom bombardment) |
Route of Synthesis (ROS)
| Conditions | Yield |
| With palladium diacetate; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; sodium t-butanolate In toluene at 80℃; for 1.5h; Arylation; | 98% |
| With sodium hydroxide; 2-(dicyclohexylphosphino)-2′-methylbiphenyl; palladium diacetate In tert-Amyl alcohol at 103℃; | 97% |
| Stage #1: bromochlorobenzene With sodium hydroxide In tert-Amyl alcohol Heating; Stage #2: benzophenone hydrazone With 2-(dicyclohexylphosphino)-2′-methylbiphenyl; palladium diacetate In tert-Amyl alcohol at 103℃; | 97% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (92%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (92%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (90%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 249.672 |
| logP | 3.971 |
| HBA | 2 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 43.09 |
| Rotatable Bond (RotB) | 2 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Pharmaceutical intermediates This compound is often used as a pharmaceutical intermediate, especially in the synthesis of anti-inflammatory, antibacterial and anticancer drugs. The amino, chlorine, and fluorine substituents in its molecular structure make it potentially biologically active. Through further chemical modification, this compound can be used to develop new biologically active drug molecules, such as quinazolines, benzimidazoles and other heterocyclic compounds with pharmacological effects. |
| Key intermediates in organic synthesis As a synthetic building block in organic synthesis, 2-amino-5-chloro-2′-fluorobenzophenone can be used to prepare a variety of heterocyclic compounds, which play an important role in drug development and material science. Its amino and keto functional groups make it suitable for a variety of chemical reactions, such as condensation reactions and cyclization reactions, to generate more complex molecules. |
| Agrochemicals In the synthesis of pesticides and herbicides, fluorine- and chlorine-containing aromatic ketone compounds have been widely studied and applied due to their efficient biological activity. This compound can be used as a lead compound for the synthesis of new agrochemical products to improve the selectivity and efficiency of crop protection agents. |
| Materials Science and Functional Materials Due to its fluorine and chlorine substituent structure, the compound has good chemical stability and heat resistance and can be used as an additive in the synthesis of functional materials, such as enhancing the properties of materials in high-performance coatings and polymers. |
| Synthesis of dyes and pigments The compound can also be used in dye chemistry as a synthetic precursor for dyes and pigments. Its unique substituents help improve the light stability and chemical resistance of dyes. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |