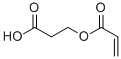2-CARBOXYETHYL ACRYLATE CAS#: 24615-84-7; ChemWhat Code: 55257
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2011/65827 | NOVEL HOLOGRAPHIC MEDIA AND PHOTOPOLYMERS | 2011 |
| US2007/225522 | Method for Producing Carboxyl Group-Containing Water-Soluble Polymer | 2007 |
| WO2005/80308 | CLEAVING OLIGOMERIC (METH)ACRYLIC ACID IN THE LIQUID PHASE AND UNDER PRESSURE | 2005 |
Physical Data
| Appearance | Transparent liquid |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 146 – 148 | 16 |
| 136 | 10 |
| 132 | 7 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.2 | 4 | 20 |
| 1.2019 | 20 | 20 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | |
| Spectrum | 1H | water-d2 | 400 |
| Chemical shifts | 1H | CDCl3 |
| Description (IR Spectroscopy) |
| Bands |
Route of Synthesis (ROS)
| Conditions | Yield |
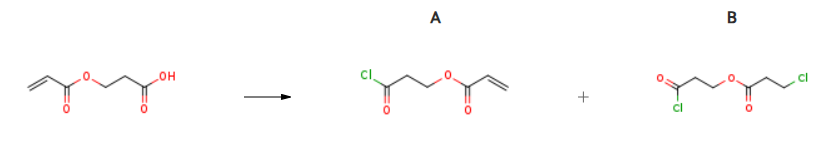
| With oxalyl dichloride; 10H-phenothiazine; 9,10-phenanthrenequinone; N,N-dimethyl-formamide In dichloromethane at 0 – 20℃; under 200 Torr; Experimental Procedure CEA from Example 7 (51 g; ~ 0.35 mole) and dimethyl formamide (DMF; 0.2 mL; 0.26 mmole) were dissolved in CH2Cl3 (100 mL). The CEA solution was added slowly (over 2 hours) to a stirred solution of oxalyl chloride (53 mL; 0.61 mole), DMF (0.2 mL; 2.6 mmole), anthraquinone (0.5 g; 2.4 mmole), phenothiazine (0.1 g, 0.5 mmole), and CH2Cl3 (75 mL) in a 500 mL round bottom flask in an ice bath at 200 mm pressure. A dry ice condenser was used to retain the CH2Cl3 in the reaction flask. After the addition was complete the reaction was stirred at room temperature overnight. The weight of reaction solution was 369 g. A sample of the CEA-Cl (Compound 7) reaction solution (124 mg) was treated with 1,4- dibromobenzene (DBB, 6.85 mg) evaporated and dissolved in CDCl3: 1H NMR (CDCl3, 400 MHz) δ 7.38 (s, 4H; DBB internal std.), 6.45 (d, IH, J = 17.4 Hz), 6.13 (dd, IH, J = 17.4, 10.4 Hz), 5.90 (d, IH, J = 10.4 Hz), 4.47 (t, 2H, J = 5.9 Hz), 3.28 (t, 2H, J = 5.9). The spectra was consistent with the desired product. There was 0.394 mole DBB for 1.0 mole CEA-Cl by integration, which gave a calculated yield of 61%. Commercially available CEA (426 g; Aldrich) was reacted with oxalyl chloride (532 mL) in a procedure similar to the one listed above. The residue CEA- Cl (490 g) was distilled using an oil bath at 1400C at a pressure of 18 mm Hg. The distillate temperature reached 980C and 150 g of distillate was collected. The distillate was redistilled at 18 mm Hg at a maximum bath temperature of 1200C. The temperature range for the distillate was 300C to 700C which gave 1 1 g of material. The distillate appeared to be 3-chloro-3-oxopropyl 3-chloropropanoate. The residue of the second distillation (125 g; 26 % of theory) was used in Example 9. | A 61% B 26% |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H302 (71.2%): Harmful if swallowed [Warning Acute toxicity, oral] H312 (57.7%): Harmful in contact with skin [Warning Acute toxicity, dermal] H314 (77.8%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] H315 (14.8%): Causes skin irritation [Warning Skin corrosion/irritation] H318 (73%): Causes serious eye damage [Danger Serious eye damage/eye irritation] H319 (14.8%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] |
| Precautionary Statement Codes | P260, P264, P264+P265, P270, P280, P301+P317, P301+P330+P331, P302+P352, P302+P361+P354, P304+P340, P305+P351+P338, P305+P354+P338, P316, P317, P321, P330, P332+P317, P337+P317, P362+P364, P363, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under the room temperature and away from light |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 144.127 |
| logP | 0.073 |
| HBA | 4 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 63.6 |
| Rotatable Bond (RotB) | 5 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Used as an excellent active diluent in UV curing coatings, adhesives, and ink systems to improve adhesion. Used as a special monomer for emulsion polymerization, adhesives, and coating resin production. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | https://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |