2-Deoxy-D-ribose CAS#: 533-67-5; ChemWhat Code: 96273
Identification
| Product Name | 2-Deoxy-D-ribose |
| IUPAC Name | (4S,5R)-5-(hydroxymethyl)oxolane-2,4-diol |
| Molecular Structure | 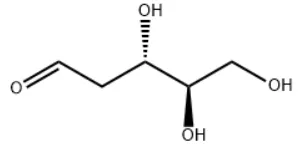 |
| CAS Registry Number | 533-67-5 |
| EINECS Number | 533-67-5 |
| MDL Number | MFCD00135904 |
| Beilstein Registry Number | 1721978 |
| Synonyms | 2-Deoxy-D-riboseD-2-deoxyribose2-deoxyribosedeoxyribose |
| Molecular Formula | C5H10O4 |
| Molecular Weight | 134.131 |
| InChI | InChI=1S/C5H10O4/c6-2-1-4(8)5(9)3-7/h2,4-5,7-9H,1,3H2/t4-,5+/m0/s1 |
| InChI Key | ASJSAQIRZKANQN-CRCLSJGQSA-N |
| Canonical SMILES | O=CCC@HC@HCO |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN118812465 | NEW BENZAMIDE DERIVATIVES Preparation method of tetrahydrofuran derivative | 2024 |
| US4861903 | Intermediates for preparing optically active carboxylic acids | 1989 |
| EP1491549 | Production of 2′-deoxynucleosides and 2′-deoxynucleoside precursors from 2-dehydro-3-deoxy-D-gluconate | 2004 |
Physical Data
| Appearance | White to off-white crystalline powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C | Solvent (Melting Point) |
| 93 – 95 | acetone, isopropyl alcohol |
| 72 – 76 | |
| 86 – 89 | |
| 77 – 84 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | water-d2 | 400 | |
| Chemical shifts | 13C | D2O | ||
| Spectrum | 1H | D2O | 43.3 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | D2O | 1715 cm**(-1) |
| Spectrum |
| Description (Mass Spectrometry) |
| gas chromatography mass spectrometry (GCMS), spectrum |
| high resolution mass spectrometry (HRMS), electrospray ionisation (ESI), liquid chromatography mass spectrometry (LCMS), spectrum |
| MALDI (Matrix assisted laser desorption ionization), time-of-flight mass spectra (TOFMS), spectrum |
| liquid chromatography mass spectrometry (LCMS), high resolution mass spectrometry (HRMS), gas chromatography mass spectrometry (GCMS), spectrum |
| Description (UV/VIS Spectroscopy) |
| Spectrum |
Route of Synthesis (ROS)
| Conditions | Yield |
| With bromine In water at 25℃; for 120h; | 88% |
| With bromine In water at 20℃; for 120h; | 78% |
| With bromine; potassium carbonate In water at 20℃; for 8h; | 70% |
| With bromine In water at 20℃; for 120h; Sealed tube; | |
| With bromine In water at 20℃; for 120h; Sealed tube; |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 134.132 |
| logP | -1.889 |
| HBA | 4 |
| HBD | 3 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 77.76 |
| Rotatable Bond (RotB) | 4 |
| Matching Veber Rules | 2 |
| Use Pattern |
| 2-Deoxy-D-ribose is widely used in DNA research, cell oxidative stress experiments, biochemical analysis, and nucleoside synthesis. It is not a product with direct industrial or pharmaceutical applications. Main Applications 1. Research Uses As a basic building block of DNA, it is often used in molecular biology research. The metabolism and role of 2-deoxy-D-ribose are often explored in studies of DNA damage and repair mechanisms. |
| Biochemical Reagents It is often used as a reducing sugar in biochemical experiments, such as testing redox reactions and free radical reactions. It is used in detection and quantification methods (for example, determining the content of deoxysugars). |
| Medical and Pharmacological Research It is used as an inducing factor in cell oxidative stress and apoptosis experiments because it promotes the generation of reactive oxygen species (ROS). Studies have used it to investigate the mechanisms of oxidative stress associated with cardiovascular disease, diabetic complications, and other conditions. |
| Synthetic Applications It can serve as an intermediate in the synthesis of certain nucleosides, nucleotides, or nucleoside analogs. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |


