2-Dicyclohexylphosphino-2′,6′-dimethoxybiphenyl CAS#: 657408-07-6; ChemWhat Code: 38351
Identification
| Product Name | 2-Dicyclohexylphosphino-2′,6′-dimethoxybiphenyl |
| IUPAC Name | dicyclohexyl-[2-(2,6-dimethoxyphenyl)phenyl]phosphane |
| Molecular Structure | 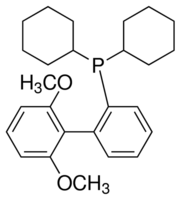 |
| CAS Registry Number | 657408-07-6 |
| MDL Number | MFCD05861611 |
| Synonyms | dicyclohexyl-(2′,6′-dimethoxybiphenyl-2-yl)-phosphane, dicyclohexyl({2′,6’‐dimethoxy‐[1,1’‐biphenyl]‐2‐yl})phosphane, 2–dicyclohexylphosphino–2′,6’–dimethoxy–1,1’–biphenyl, dicyclohexyl (2’,6’-dimethoxy-[1,1’-biphenyl]-2-yl)phosphine, dicyclohexyl(2’,6’-dimethoxy-[ 1,1’-biphenyl]-2-yl)phosphine, tert 2-dicyclohexylphosphino-2′,6′-dimethoxybiphenyl-butoxycarbonyl, (2’,6’-dimethoxy-[1,1’-biphenyl]-2-yl)dicyclohexylphosphine CAS#: 657408-07-6;CAS Number: 657408-07-6;CAS No: 657408-07-6 |
| Molecular Formula | C26H35O2P |
| Molecular Weight | 410.529 |
| InChI | InChI=1S/C26H35O2P/c1-27-23-17-11-18-24(28-2)26(23)22-16-9-10-19-25(22)29(20-12-5-3-6-13-20)21-14-7-4-8-15-21/h9-11,16-21H,3-8,12-15H2,1-2H3 |
| InChI Key | VNFWTIYUKDMAOP-UHFFFAOYSA-N |
| Canonical SMILES | COc1cccc(c1c2ccccc2P(C3CCCCC3)C4CCCCC4)OC |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2009/143586 | Method for producing alkyl-substituted aromatic and heteroaromatic compounds by cross-coupling alkyl boronic acids with aryl-or heteroaryl-halogenides or sulfonates under Pd catalysis in the presence of a ligand | 2009 |
| US2006/173186 | Transition-metal-catalyzed carbon-nitrogen and carbon-carbon bond-forming reactions | 2006 |
Physical Data
| Appearance | White crystal |
| Sensitivity | Air Sensitive |
| Melting Point, °C | Solvent (Melting Point) |
| 162 – 163 | |
| 163 – 165 | acetone |
| 162 – 162.5 | acetone |
| Description (Association (MCS)) | Partner (Association (MCS)) |
| NMR spectrum of the complex | CuCl |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Spectrum | 1H | |||
| Spectrum | 31P | chloroform-d1 | 243 | |
| Chemical shifts, Spectrum | 1H | benzene-d6 | 400 | |
| Chemical shifts, Spectrum | 31P | benzene-d6 | 161 | |
| Chemical shifts | 31P | 202.5 | ||
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 19.64 | 600 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 21.04 | 151 |
| Chemical shifts, Spectrum | 31P | chloroform-d1 | 26.84 | 81 |
| Chemical shifts | 1H | chloroform-d1 | 400 | |
| Chemical shifts | 31P | chloroform-d1 | 162 | |
| Chemical shifts | 13C | benzene-d6 | 75 | |
| Chemical shifts | 31P | benzene-d6 | 121 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Original Text (IR Spectroscopy) | Signals, cm-1 |
| in KBr | IR (KBr): υ=3000, 2923, 2851, 1588, 1471, 1442, 1428, 1108 cm-1 | 3000, 2923, 2851, 1588, 1471, 1442, 1428, 1108 | |
| Bands | solid |
| Description (Mass Spectrometry) |
| HRMS (High resolution mass spectrometry), TOFMS (Time of flight mass spectrum), EI (Electron impact), Spectrum |
Route of Synthesis (ROS)
| Conditions | Yield |
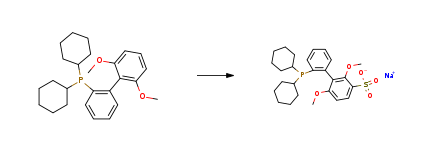
| Stage #1: dicyclohexyl-(2′,6′-dimethoxybiphenyl-2-yl)-phosphane With sulfuric acid In dichloromethane at 0 – 40℃; for 24h; Stage #2: With sodium hydroxide In dichloromethane; water at 0℃; pH=~ 7.0; Cooling with ice; Experimental Procedure To an oven-dried 25 mL round bottom flask equipped with a Teflon-coated magnetic stir bar and rubber septum was added 2-dicyclohexylphosphino-2’6’dimethoxybiphenyl (5.13 g, 12.5 mmol) and CH2Cl2 (5 mL). The solution was cooled to 0° C. using an ice/water bath and then concentrated H2SO4 (32.5 mL, 625 mmol) was added dropwise. The solution slowly turned yellow in color. The solution was heated to 40° C. in a preheated oil bath and was allowed stir for 24 h. At this time it was cooled to 0° C. using an ice/water bath and crushed ice (50 g) was added. The solution turned cloudy and white in color. An aqueous solution of NaOH (6.0 M, 200 mL) was then added dropwise to the cooled solution until it became neutral (pH 7.0 as judged by pH paper). The aqueous solution was extracted with CH2Cl2 (3×300 mL) and concentrated under reduced pressure to give a light yellow solid. The crude material was then dissolved in a minimum amount of cold methanol (20 mL), filtered and concentrated (this cycle was repeated) to give sodium 2-dicyclohexylphosphino-2′,6′-dimethoxybiphenyl-3′-sulfonate (2) as a light yellow solid (6.35 g, 99%). Mp=165° C. (turned red, dec.) 1H NMR (400 MHz, CD3OD) δ: 7.88 (d, 1H, J=8.8 Hz), 7.60 (m, 1H), 7.36 (m, 2H), 7.22 (m, 1H), 6.76 (d, 1H, J=8.8 Hz) 3.70 (s, 3H), 3.39 (s, 3H), 1.14-2.01 (m, 22H). 13C NMR (125 MHz, CD3OD) δ: 161.3, 157.1, 143.3, 142.9, 137.9, 137.8, 133.7, 133.6, 133.3, 133.2, 131.9, 130.0, 129.3, 128.0, 127.9, 127.8, 105.9, 61.6, 56.1, 50.0, 37.0, 36.9, 34.8, 34.6, 31.7, 31.6, 31.5, 31.2, 31.0, 30.8, 30.7, 28.9, 28.8, 28.7, 28.4, 28.34, 28.31, 28.2, 27.8, 27.7. 31P NMR (162 MHz, CD3OD) δ: -8.02. IR (neat, cm-1): 3453, 2925, 2849, 1577, 1462, 1448, 1400, 1229, 1191, 1099, 1053, 736. Anal. Calcd for C26H34NaO5PS: C, 60.92; H, 6.69. Found: C, 60.40; H, 6.85. 1H NMR (d4-MeOH/D2O) is shown in FIG. 7. 31P NMR (d4-MeOH) is shown in FIG. 7. | 99% |
| Stage #1: dicyclohexyl-(2′,6′-dimethoxybiphenyl-2-yl)-phosphane With sulfuric acid In dichloromethane at 0 – 40℃; for 24.7h; Inert atmosphere; Stage #2: With sodium hydroxide In dichloromethane; water at 0℃; pH=13; | 62% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H302+H312+H332 (11.11%): Harmful if swallowed, in contact with skin or if inhaled [Warning Acute toxicity, oral; acute toxicity, dermal; acute toxicity, inhalation] H302 (55.56%): Harmful if swallowed [Warning Acute toxicity, oral] H312 (22.22%): Harmful in contact with skin [Warning Acute toxicity, dermal] H315 (88.89%): Causes skin irritation [Warning Skin corrosion/irritation] H317 (33.33%): May cause an allergic skin reaction [Warning Sensitization, Skin] H319 (88.89%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H332 (22.22%): Harmful if inhaled [Warning Acute toxicity, inhalation] H335 (55.56%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P270, P271, P272, P280, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P312, P321, P322, P330, P332+P313, P333+P313, P337+P313, P362, P363, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| Storage | The product will be slowly oxidized in the air, stored for a long time, it needs to be sealed and dried below 0℃ |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 410.536 |
| logP | 8.007 |
| HBA | 0 |
| HBD | 0 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 32.05 |
| Rotatable Bond (RotB) | 6 |
| Matching Veber Rules | 2 |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Dose |
| 4.5 | inhibition rate(of cell proliferation) | 100 | % | 600 μM | |
| 3.82 | inhibition rate(of cell proliferation) | < | 50 | % | 150 μM |
| Use Pattern |
| Palladium-catalyzed Suzuki-Miyaura cross-coupling reaction between Boc-protected aminomethyl trifluoroborate and aryl chloride or heteroaryl chloride to form the corresponding Aminomethyl aromatic hydrocarbons. |
| Palladium-catalyzed Suzuki-Miyaura cross-coupling reaction between 4-methyl substituted piperidinyl zinc reagent and different aryl or heteroaryl iodides to form various substituted piperidines. |
| Intramolecular Suzuki-Miyaura coupling during the multi-step synthesis of riccardin C to form an 18-membered macrocycle. Used with palladium to produce a highly active catalyst for C-N bond formation. |
| Highly versatile ligand for Suzuki-Miyaura coupling reaction; aryl chloride, sterically hindered diaryl, heterodiaryl compounds. |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |