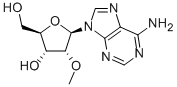
2′-O-Methyladenosine CAS#: 2140-79-6; ChemWhat Code: 96875
Identification
Physical Data
| Appearance | White powder |
| Melting Point, °C | Solvent (Melting Point) |
| 204 – 206 | |
| 202 – 203 | ethanol |
| 200 – 201 | |
| 198 – 201 | |
| 203 – 207 |
| Density, g·cm-3 |
| 1.482 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | 25 | ||
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 25 | 500 |
| Chemical shifts | 13C | dimethylsulfoxide-d6 | 125 | |
| Chemical shifts | 1H | CD3OD | 500 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | neat (no solvent, solid phase) | |
| Bands | potassium bromide |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Absorption maxima | H2O | 259 | 14000 | |
| Absorption maxima | 259 | 400 | ||
| Absorption maxima | H2O | Remark: pH 7 | 259 | 13804 |
Route of Synthesis (ROS)
| Conditions | Yield |
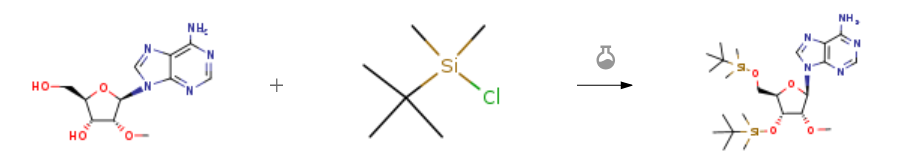
| With 1H-imidazole In pyridine at 65℃; for 12h; silylation; | 93% |
| With 1H-imidazole; dmap In N,N-dimethyl-formamide at 20℃; for 28h; | 83% |
| With pyridine; 1H-imidazole at 20℃; for 15h; Experimental Procedure 117-130 Preparation of compound 7-2: To a solution of 7-1 (15.0 g, 53.3 mmol) in dry pyridine (150 mL) was added TBSC1 (20.0 g, 133.3 mmol) and Imidazole (10.8 g, 159.9 mmol). The mixture was stirred at r.t. for l5h. TLC showed 7-1 was consumed completely. The reaction mixture was concentrated in vacuo to give residue. The residue was quenched with DCM (500 mL). The DCM layer was washed with H2O (1 L*2) 2 times and brine. The DCM layer concentrated in vacuo to give crude 7-2 (27.2 g, 53.3 mmol) as a yellow oil. The crude 7-2 was used in next step directly. ESI-LCMS m/z 510.5 [M+H]+. | 99% |
Safety and Hazards
No data available
Other Data
No data available
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 281.271 |
| logP | -1.327 |
| HBA | 9 |
| HBD | 3 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 128.54 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Quantitative Results | ||
| 1 of 25 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Novel adenosine derivatives and pharmaceutical composition containing them as an active ingredient | |
| 2 of 25 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Methylation of Adenosine and Related Nucleosides with Trimethylselenonium Hydroxyde, and Regiospecific Effects of Copper(II) Ions | |
| 3 of 25 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Nucleic acid related compounds. 36. Synthesis of the 2′-O-methyl and 3′-O-methyl ethers of guanosine and 2-aminoguanosine and correlation of O’-methylnucleoside 13C nmr spectral shifts | |
| 4 of 25 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | The first synthesis of herbicidin B. stereoselective construction of the tricyclic undecose moiety by a conformational restriction strategy using steric repulsion between adjacent bulky silyl protecting groups on a pyranose ring | |
| 5 of 25 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Inhibitory actvity of the compound against Fatty acid amide hydrolase in 0.1 M sodium phosphate, pH 8.0 | |
| 6 of 25 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Recognition and detection of 8-oxo-rG in RNA using the DNA/OMeRNA chimera probes containing fluorescent adenosine-diazaphenoxazine analog | |
| 7 of 25 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Nucleoside derivatives as inhibitors of RNA-dependent RNA viral polymerase | |
| 8 of 25 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | RNAi agents and compositions for inhibiting expression of apolipoprotein C-III (APOC3) | |
| 9 of 25 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | MODIFIED SHORT INTERFERING NUCLEIC ACID (SINA) MOLECULES AND USES THEREOF | |
| 10 of 25 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Adolescent alcohol exposure changes RNA modifications in adult brain by mass spectrometry-based comprehensive profiling analysis |
| Use Pattern |
| 2′-O-Methyladenosine CAS#: 2140-79-6 (N-EPSILON-L-LYSINE METHYL ESTER HYDROCHLORIDE SALT and it is used in IVD. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |