2-Sulfobenzoic anhydride CAS#: 81-08-3; ChemWhat Code: 58468
Identification
| Product Name | 2-Sulfobenzoic anhydride |
| IUPAC Name | 1,1-dioxo-2,1λ6-benzoxathiol-3-one |
| Molecular Structure | 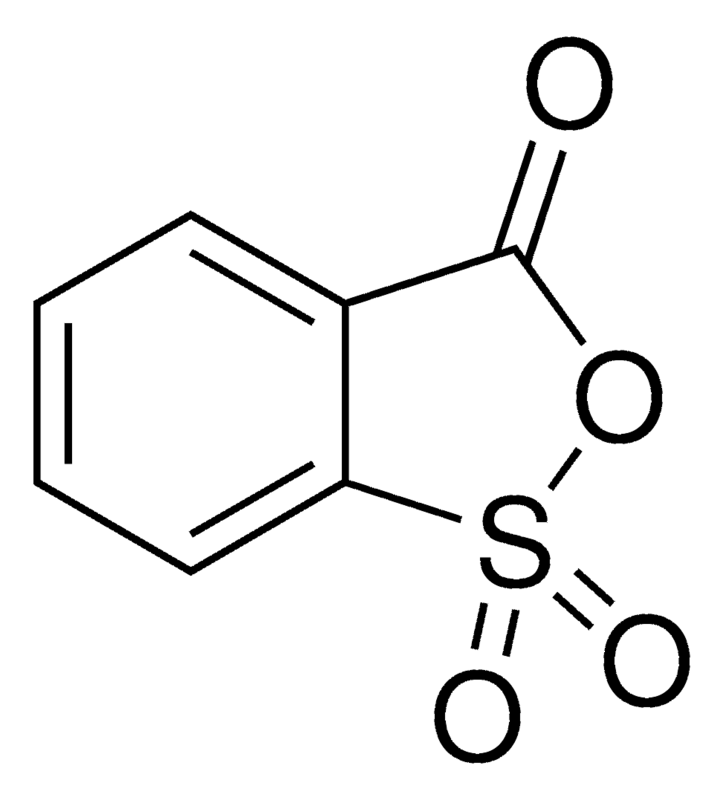 |
| CAS Registry Number | 81-08-3 |
| EINECS Number | 201-322-6 |
| MDL Number | MFCD00005879 |
| Beilstein Registry Number | 139893 |
| Synonyms | 2-sulfobenzoic acid cyclic anhydrideo-sulfobenzoic anhydride |
| Molecular Formula | C7H4O4S |
| Molecular Weight | 184.17 |
| InChI | InChI=1S/C7H4O4S/c8-7-5-3-1-2-4-6(5)12(9,10)11-7/h1-4H |
| InChI Key | NCYNKWQXFADUOZ-UHFFFAOYSA-N |
| Canonical SMILES | O=C1OS(=O)(=O)c2ccccc21 |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN112239453 | Preparation method of acyl sulfonate compounds | 2021 |
| CN110028487 | Preparation method of thiochromone compound | 2019 |
Physical Data
| Appearance | Off-white solid |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C | Solvent (Melting Point) |
| 121 | |
| 128 – 129 | |
| 224 – 226 | |
| 130 | |
| 128 | benzene |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 184 – 186 | 18 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
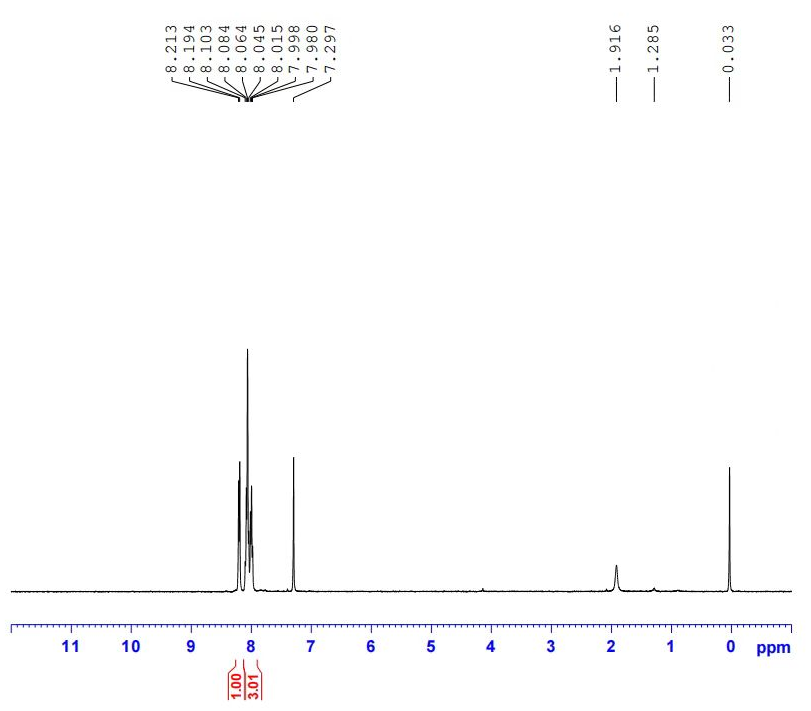
| Chemical shifts | 1H | chloroform-d1 | 500 |
| Chemical shifts | 1H | dimethylsulfoxide-d6, chloroform-d1 | |
| Chemical shifts | 1H | dimethylsulfoxide-d6, chloroform-d1 | 300 |
| Chemical shifts | 13C | dimethylsulfoxide-d6, chloroform-d1 | 75 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | potassium bromide |
| Description (Mass Spectrometry) |
| LCMS (Liquid chromatography mass spectrometry), Spectrum |
| EI (Electron impact), GCMS (Gas chromatography mass spectrometry), Spectrum |
| fragmentation pattern |
Route of Synthesis (ROS)
| Conditions | Yield |
| In xylene for 0.00416667h; Microwave irradiation; | 77% |
| With Zn0.95*Ti0.05O In neat (no solvent) at 140℃; for 2h; Green chemistry; | 58% |
| at 130 – 135℃; |
Safety and Hazards
| Pictogram(s) |     |
| Signal | Danger |
| GHS Hazard Statements | H302 (11.1%): Harmful if swallowed [Warning Acute toxicity, oral] H312 (11.1%): Harmful in contact with skin [Warning Acute toxicity, dermal] H314 (11.1%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] H315 (88.9%): Causes skin irritation [Warning Skin corrosion/irritation] H318 (11.1%): Causes serious eye damage [Danger Serious eye damage/eye irritation] H319 (88.9%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H332 (11.1%): Harmful if inhaled [Warning Acute toxicity, inhalation] H335 (66.7%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P260, P261, P264, P264+P265, P270, P271, P280, P301+P317, P301+P330+P331, P302+P352, P302+P361+P354, P304+P340, P305+P351+P338, P305+P354+P338, P316, P317, P319, P321, P330, P332+P317, P337+P317, P362+P364, P363, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 184.172 |
| logP | 0.149 |
| HBA | 4 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 68.82 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Use Pattern |
| 2-Sulfobenzoic anhydride (CAS# 81-08-3), is a chemical intermediate with a range of applications, particularly in organic synthesis and industrial processes. Below are some of its key uses : 1. Chemical Intermediate It serves as a precursor in the synthesis of sulfonated compounds, which are widely used in the production of dyes, pigments, and surfactants. 2. Pharmaceutical Synthesis Utilized in the preparation of certain pharmaceutical compounds, particularly sulfonamides or derivatives that require a sulfonic acid functional group. 3. Catalyst or Reagent Often used as a reagent in chemical reactions that require a sulfonylating agent. 4. Material Science Can be involved in the modification of polymers or the production of specialty resins. 5. Research Applications Frequently used in academic and industrial research to develop novel materials or explore reaction mechanisms involving anhydride or sulfonic acid groups. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |