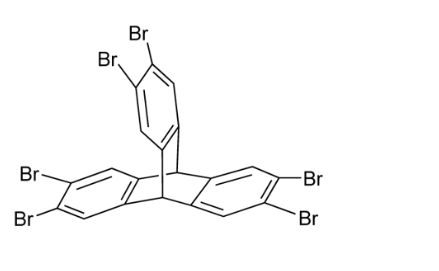
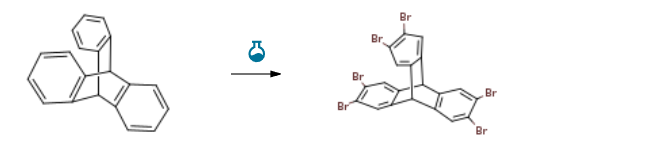
2,3,6,7,12,13-Hexabromotriptycene CAS#: 55805-81-7; ChemWhat Code: 1491291
Identification
Physical Data
| Melting Point, °C | Solvent (Melting Point) |
| 350 | acetone |
| 360 – 362 | ethanol |
| 362 – 365 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d1 | ||
| Chemical shifts | 1H | chloroform-d1 | 400 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | ||
| Chemical shifts, Spectrum | 13C | chloroform-d1 | ||
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 26.84 | 500 |
| Chemical shifts | 1H | chloroform-d1 | 26.84 | 400 |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 300 |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum | dichloromethane | 229, 285, 295 |
Route of Synthesis (ROS)
| Conditions | Yield |
| With bromine; iodine; iron In chloroform at 79℃; Experimental Procedure 4 Synthesis of compound 4 Add triptycene (1g, 3.9mmol), liquid bromine (1.49mL, 29mmoL), iron powder (73.7mg), iodine (144.9mg), 80mL chloroform, reflux at 79°C, and check the reaction progress by nuclear magnetic After the reaction is completed, it is cooled to room temperature, filtered, and the solvent is removed in vacuo to obtain hexabromotriptycene 4 (2.5 g) as a white solid with a yield of 90%. | 90% |
| With bromine; iodine; iron In chloroform | 90% |
| With bromine; iron In 1,2-dichloro-ethane for 6h; Reflux; | 80% |
Safety and Hazards
No data available
Other Data
| Transportation | Under the room temperature and away from light |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 727.708 |
| logP | 10.032 |
| HBA | 0 |
| HBD | 0 |
| Matching Lipinski Rules | 2 |
| Veber rules component | |
| Polar Surface Area (PSA) | 0 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Due to its unique structure and the presence of bromine substituents, 2,3,6,7,12,13-Hexabromotriptycene might have potential applications in electronic materials. For example, it could be used in organic optoelectronic devices or semiconductor materials. Being an organic compound with a distinctive structure, 2,3,6,7,14,15-hexabromotriphenylene could be employed as a starting material or intermediate in various chemical reactions and synthesis routes. Bromine compounds are commonly used as flame retardants to slow down or prevent the combustion of materials. 2,3,6,7,14,15-hexabromotriphenylene may find applications as a flame retardant in certain industries. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| WatsonChem Advanced Chemical Materials | https://www.watsonchem.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |