2,7-diaminopyrene-4,5,9,10-tetraone CAS#: 2459874-51-0; ChemWhat Code: 1491396
Identification
| Product Name | 2,7-diaminopyrene-4,5,9,10-tetraone CAS 2459874-51-0 |
| IUPAC Name | 2,7-diaminopyrene-4,5,9,10-tetrone |
| Molecular Structure | 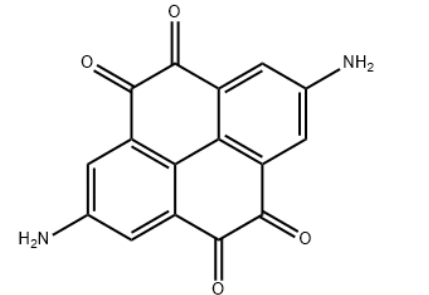 |
| CAS Registry Number | 2459874-51-0 |
| Synonyms | 2,7-Diaminopyrene-4,5,9,10-tetraone 2459874-51-0 SCHEMBL28419032 MFCD35093308 CS-0379117 H39228 |
| Molecular Formula | C16H8N2O4 |
| Molecular Weight | 292.24 |
| InChI | InChI=1S/C16H8N2O4/c17-5-1-7-11-8(2-5)15(21)16(22)10-4-6(18)3-9(12(10)11)14(20)13(7)19/h1-4H,17-18H2 |
| InChI Key | IKBICLVLEOZSQF-UHFFFAOYSA-N |
| SMILES | C1=C(C=C2C3=C1C(=O)C(=O)C4=C3C(=CC(=C4)N)C(=O)C2=O)N |
Physical Data
No data available
Spectra
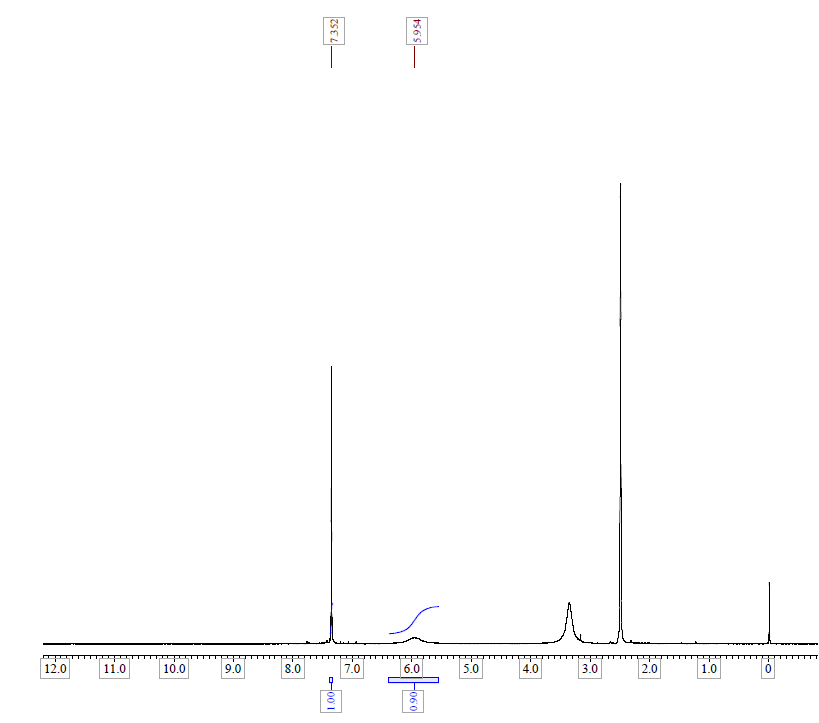
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 24.84 |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | 24.84 |
| Chemical shifts, Spectrum | 1H |
| Description (IR Spectroscopy) |
| Bands, Spectrum |
| Bands, Spectrum |
| Bands |
| Bands, Spectrum |
Route of Synthesis (ROS)
| Conditions | Yield |
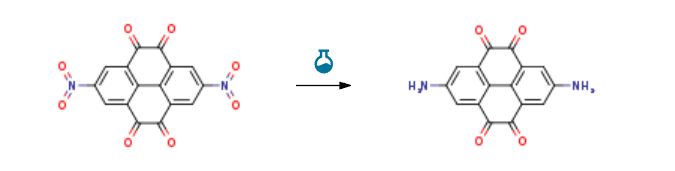
| With sodium dithionite; sodium hydroxide In water at 50℃; for 0.25h; Experimental Procedure 1.3 3) Synthesis of 2,7-diamino-4,5,9,10-tetrahydropyrene-4,5,9,10-tetrone (I-2): Put 2,7-dinitro-4,5,9,10-tetrahydropyrene-4,5,9,10-tetrone(III, 1g, 2.8mmol) in a 500mL two-necked flask, add sodium hydroxide (8.9g, 224mmol), water (150mL ) And sodium dithionite (4.4g, 25.5mmol),Heat to 50 in an oil bath, stir for 15min,The reaction mixture was poured into saturated ammonium chloride solution (500 mL), filtered and washed with water until the filtrate was colorless to obtain a black powder, which was dried under vacuum at room temperature overnight.To obtain a crude product, the crude product (457mg), dichlorodicyanobenzoquinone (1.5g) and methanol (30mL) were mixed,Stir for 15h in 35 oil bath,The reaction solution was diluted with ethyl acetate (60 mL), filtered with suction,Washing with ethyl acetate gave a black solid (442 mg, yield 54%). | 54% |
| With sodium sulfide In N,N-dimethyl-formamide at 80℃; Inert atmosphere; | 314 mg |
| With sodiumsulfide nonahydrate In N,N-dimethyl-formamide at 80℃; Inert atmosphere; | 70 % |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Other Data
| Transportation | Under the room temperature and away from light |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 292.251 |
| logP | 0.62 |
| HBA | 6 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 120.32 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Use Pattern |
| The versatile nature of 2,7-diaminopyrene-4,5,9,10-tetraone makes it a valuable compound with promising applications in fluorescent probes, organic optoelectronic materials, chemical synthesis, and biomedical research. 2,7-diaminopyrene-4,5,9,10-tetraone finds extensive applications in the field of fluorescent probes and dyes. Its amino and cyclohexanone functional groups enable specific interactions with target molecules, facilitating detection and imaging of specific molecules within biological systems. It can be used for cell fluorescence labeling, fluorescent sensors, and bioimaging. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |