(2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester CAS#: 401564-36-1; ChemWhat Code: 740740
Identification
| Product Name | (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester |
| IUPAC Name | (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester |
| Molecular Structure | 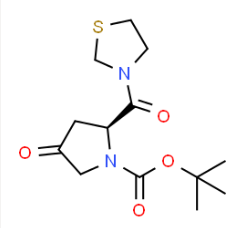 |
| CAS Registry Number | 401564-36-1 |
| EINECS Number | No data available |
| MDL Number | MFCD22665915 |
| Beilstein Registry Number | No data available |
| Synonyms | 3-Pyridinamin;3-Pyridinamine;3-Pyridinamine;pyridin-3-amine;T6NJ CZ;3- Aminopyridine;3-Amino-pyridine;3-pyridylamine;Amino-3 pyridine;m-Aminopyridine;MS/MS-1064463;Pyridin-3-ylamine;Pyridine, 3-amino-;β-Aminopyridine 462-08-8 CAS#: 401564-36-1 CAS: 401564-36-1 CAS No.: 401564-36-1 |
| Molecular Formula | C13H20N2O4S |
| Molecular Weight | 300.374 |
| InChI | InChI=1S/C13H20N2O4S/c1-13(2,3)19-12(18)15-7-9(16)6-10(15)11(17)14-4-5-20-8-14/h10H,4-8H2,1-3H3/t10-/m0/s1 |
| InChI Key | ULXKZRPRLJGLDM-JTQLQIEISA-N |
| Canonical SMILES | CC(C)(C)OC(=O)N1CC(=O)C[C@H]1C(=O)N2CCSC2 |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| JP2019/147763 | Production of compound prolinamide (by machine translation) | 2019 |
| CN103649055 | For the preparation of pyrazole derivatives (by machine translation) | 2016 |
| WO2015/19238 | PROCESS FOR THE PREPARATION OF N-PROTECTED (5S)-5-(1,3-THIAZOLIDIN-3-YLCARBONYL)PYRROLIDIN-3-ONE | 2015 |
| WO2015/63709 | PROCESS FOR THE PREPARATION OF 1-(3-METHYL-1-PHENYL-1H-PYRAZOL-5-YL)PIPERAZINE | 2015 |
Physical Data
| Appearance | White Powder |
| Solubility | No data available |
| Flash Point | 251ºC |
| Refractive index | No data available |
| Sensitivity | No data available |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.305 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz | Original Text (NMR Spectroscopy) | Comment (NMR Spectroscopy) |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 500 | ||
| Chemical shifts | 1H | chloroform-d1 | |||
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 500 | ||
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 500 | ||
| Chemical shifts | 1H | CDCl3 | 300 | ||
| 1H | d(4)-methanol | 300 | 1H NMR (300 MHz, methanol-d4): δ ppm 5.07 (d, 1H), 4.80 (m, 1H), 4.57-4.68 (m, 2 H), 4.45 (m, 1H), 3.85 (d, 2H), 3.78 (m, 2H), 3.17 (t, 1H), 3.05 (m, 2 H), 2.44-2.49 (d, 1H), 1.47 (s, 9H) | Signals given | |
| 1H | d(4)-methanol | 300 | 1H NMR (300 MHz, methanol-d4): δ ppm 5.07 (d, 1H), 4.80 (m, 1H), 4.57-4.68 (m, 2H), 4.45 (m, 1H), 3.85 (d, 2H), 3.78 (m, 2H), 3.17 (t, 1H), 3.05 (m, 2H), 2.44-2.49 (d, 1H), 1.47 (s, 9H) | Signals given | |
| 1H | chloroform-d1 | 1H-NMR(CDCl3)δ 1.47(9H,s), 2.45-2.57(1H,m), 2.70-2.93(1H,m), 2.97-3.22(2H,m), 3.66-3.78(0.6H,m), 3.80-4.10(3H,m), 4.28-4.38(0.4H,m), 4.45-5.08(3H,m). | Signals given |
| Description (Mass Spectrometry) | Comment (Mass Spectrometry) | Peak |
| ESI (Electrospray ionisation), CI (Chemical ionization) | Molecular peak | 299 m/z |
Route of Synthesis (ROS)
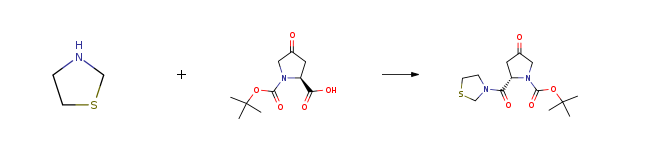
| Conditions | Yield |
| With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; N-ethyl-N,N-diisopropylamine In ethyl acetate at 2 – 7℃; for 2h; Large scale; Experimental Procedure 5 Preparation of 3-[(2S)-1-tert-butoxycarbonyl-4-oxopyrrolidin-2-ylcarbonyl]thiazolidine To (2S)-1-tert-butoxycarbonyl-4-oxopyrrolidine-2-carboxylic acid (Compound 8a) (60.0 kg),thiazolidine (30.3 kg) and N,N-diisopropylethylamine ( 118kg) in ethyl acetate and propylphosphonic anhydride (595kg) (cyclic trimer) was added 28w% at 2 -7 °C in ethyl acetate (446kg) was added and the reaction mixture was 2 -4 °C stirred for 2 hours. To this reaction mixture was added 15w% aqueous citric acid (600kg) for distribution, and the aqueous layer with ethyl acetate (271kg) extract. The ethyl acetate layer obtained was mixed, and sequentially washed with 10w% aqueous solution of diammoniumphosphate (600kg) and water (300kg). The ethyl acetate layer was concentrated to a residual volume 300L, n-heptane (739kg) at 23 -25 °C added, and the mixture wasstirred at 23 -25 °C 1 hour and stirred at 1 -5 °C. The precipitated crystals were collected by filtration, with n-heptane (164 kg) were washed and driedunder reduced pressure to yield 3-[(2S)-1-tert-butoxycarbonyl-4-oxopyrrolidin-2-ylcarbonyl]thiazolidine (compound 9a) (67.8 kg, yield 86 %) | 86% |
| Stage #1: N-tert-butoxycarbonyl-4-oxo-L-proline With pivaloyl chloride; N-ethyl-N,N-diisopropylamine In ethyl acetate at 10℃; for 0.5h; Large scale; Stage #2: 1,3-thiazolidine In ethyl acetate at 0 – 10℃; for 1h; Reagent/catalyst; Temperature; Solvent; Large scale; Experimental Procedure 1; 1-10; 13 Example 1 Preparation of 3-[(2S) -1-t-butoxycarbonyl-4-oxopyrrolidin-2-ylcarbonyl] thiazolidine (Compound 2a) (2S) -1-t-butoxycarbonyl-4-oxopyrrolidine-2-carboxylic acid (Compound 3a)(250.0 kg), N, N-diisopropylethylamine (DIPEA) (141 kg) in ethyl acetate (2242.5 kg) was added with pivaloyl chloride (131.5 kg) at 10 ° C. or lower, and the reaction mixture was added. The mixture was stirred at 10 ° C. or lower for 30 minutes.After thiazolidine (97.2 kg) was added to the reaction mixture at 10 ° C. or lower,The reaction mixture was stirred at 0-10 ° C. for 1 hour.To this reaction mixture, water (500.0 kg) was added for liquid separation,Ethyl acetate layer was prepared with diammonium hydrogen phosphate aqueous solution (prepared from 144.0 kg ammonium hydrogen phosphate and water (750.0 kg)) and saline (prepared from salt (75.0 kg) and water (425.0 kg)). Washed sequentially.After concentrating the ethyl acetate layer to a residual amount of 1250 L,2-Propanol (976.3 kg) was added and concentrated again.After the remaining amount was 1000 L, n-heptane (1368 kg) was added at 40 to 45 ° C., and the mixture was stirred at -5 ° C. or lower for 1 hour.The precipitated crystals were collected by filtration and washed with n-heptane (684.0 kg).2-Propanol (429.6 kg) was added to the obtained crystals, n-heptane (1521.9 kg) was added at 40 to 45 ° C., and the mixture was stirred at -5 ° C. or lower for 1 hour.The precipitated crystals are collected by filtration, washed with n-heptane (769.8 kg), and then dried under reduced pressure.3-[(2S) -1-t-butoxycarbonyl-4-oxopyrrolidin-2-ylcarbonyl] thiazolidine283.1 kg of (Compound 2a) was obtained.(Yield 86%) | 86% |
| Stage #1: N-tert-butoxycarbonyl-4-oxo-L-proline With dicyclohexyl-carbodiimide In toluene at -10 – -5℃; Stage #2: 1,3-thiazolidine With dmap In toluene at -6 – 5℃; for 1.33333h; Experimental Procedure 3 Example 3: Preparation of tert-Butyl (2S)-4-oxo-2-(1.3-thiazolidin-3- ylcarbonyl)pyrrolidine-1-carboxylate(Formula III) A solution of DCC (107.8 g) in toluene (300 mL) was added to a solution of 1-(tert-butoxycarbonyl)-4-oxo-L-proline (prepared according to the process of Example 2; 100 g) in toluene (900 mL) at -5°C to – 10°C, and the reaction mixture was stirred for 30 minutes at the same temperature. Dimethylaminopyridine (1 g) and thiazolidine (46.7 g) were slowly added to the reaction mixture at a temperature of about -6°C to -2°C over a period of about 15 to 20 minutes. The reaction mixture was allowed to warm to a temperature of 0°C to 5°C, and stirred at the same temperature for 60 minutes. When the reaction was complete, the reaction mixture was quenched with deionized water (20 mL), and stirred at 20°C to 25°C for 30 minutes. The resulting mixture was filtered through a Hyflo bed. The filtrate was washed with aqueous sodium bicarbonate solution (50 g sodium carbonate in 500 mL deionized water). The organic layer was separated and washed with an aqueous solution of sodium chloride (50 g sodium chloride in 500 mL deionized water). Activated carbon (10 g) was added to the organic layer, and the reaction mixture was stirred at 25°C to 30°C for 30 minutes. The reaction mixture was filtered through a Hyflo bed, and concentrated at a temperature of 50°C under reduced pressure. The residue obtained was dissolved in ethyl acetate (200 mL) at 50°C to 55°C. Hexanes (800 mL) were added at 50°C to 55°C over a period of 1 to 2 hours. The reaction mixture was further cooled to a temperature of 0°C to 5°C, and stirred at the same temperature for 3 hours. The reaction mixture was filtered to obtain a solid. The solid was washed with a pre-cooled (0°C to 5°C) mixture of ethyl acetate (80 mL) and hexanes (320 mL), and dried at a temperature of 40°C to 45°C under reduced pressure to obtain tert-Butyl (2S)-4-oxo-2-(1.3-thiazolidin-3- ylcarbonyl)pyrrolidine-1-carboxylate. Yield: 81.7% | 81.7% |
| With dmap; dicyclohexyl-carbodiimide In toluene at -6 – 5℃; for 1.33333h; | 81.7% |
| Stage #1: N-tert-butoxycarbonyl-4-oxo-L-proline With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In toluene at 0℃; for 3h; Large scale; Stage #2: 1,3-thiazolidine With dmap at 0℃; for 2h; Reagent/catalyst; Large scale; | 50kg |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] [Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 300.379 |
| logP | 0.41 |
| HBA | 6 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 92.22 |
| Rotatable Bond (RotB) | 5 |
| Matching Veber Rules | 2 |
| Use Pattern |
| (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester CAS#: 401564-36-1 is used as General chemicals |
| (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester CAS#: 401564-36-1 is used as intermediate for producing tenerigliptin. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

