3-BOC-3-METHYLAMINOAZATIDINE CAS#: 577777-20-9; ChemWhat Code: 60569
Identification
| Product Name | 3-BOC-3-METHYLAMINOAZATIDINE |
| IUPAC Name | tert-butyl N-(azetidin-3-yl)-N-methylcarbamate |
| Molecular Structure | 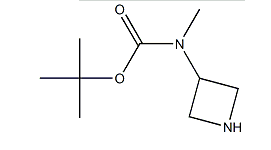 |
| CAS Registry Number | 577777-20-9 |
| MDL Number | MFCD08752583 |
| Synonyms | azetidin-3-yl-N-methylcarbamic acid tert-butyl ester, azetidin-3-ylmethylcarbamic acid tert-butyl ester, tert-butyl N-(azetidin-3-yl)-N-methylcarbamate, tert-butyl azetidin-3-yl(methyl)carbamate, 3-Boc-3-methylaminoazetidine, 3-(N-Boc-N-methylamino)-azetidine, 3-Boc-3-methylaminoazatidine;CAS Number: 577777-20-9;CAS NO.:577777-20-9 |
| Molecular Formula | C9H18N2O2 |
| Molecular Weight | 186.25 |
| InChI | InChI=1S/C9H18N2O2/c1-9(2,3)13-8(12)11-6-7-4-10-5-7/h7,10H,4-6H2,1-3H3,(H,11,12) |
| InChI Key | MOLUHRBHXXGWDP-UHFFFAOYSA-N |
| Canonical SMILES | CC(C)(C)OC(=O)NCC1CNC1 |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2019/76974 | SYNTHESIS OF 4-AMINOPYRIMIDINE COMPOUNDS | 2019 |
| US2014/315888 | NOVEL HETEROCYCLIC DERIVATIVES AND THEIR USES | 2014 |
| WO2005/54239 | 2-AMINOPYRIMIDINE DERIVATIVES | 2005 |
Physical Data
| Appearance | Off-white to white powder |
| Solubility | Soluble in water. (5 g/100ml) |
| Boiling Point | 247.9±29.0 °C(Predicted) |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz | Original Text (NMR Spectroscopy) | Signals, ppm |
| 1H | d(4)-methanol | 300 | 1H NMR (300 MHz, CD3OD)δ: 1.44 (s, 9H), 2.88 (s, 3H), 3.56 (m, 2H), 3.71 (m, 2H), 4.75 (m, 1 H). | ||
| 31P | d(4)-methanol | 300 | 1H NMR (300 MHz, CD3OD) δ: 1.44 (s, 9H), 2.88 (s, 3H), 3.56 (m, 2H), 3.71 (m, 2H), 4.75 (m, 1 H). | ||
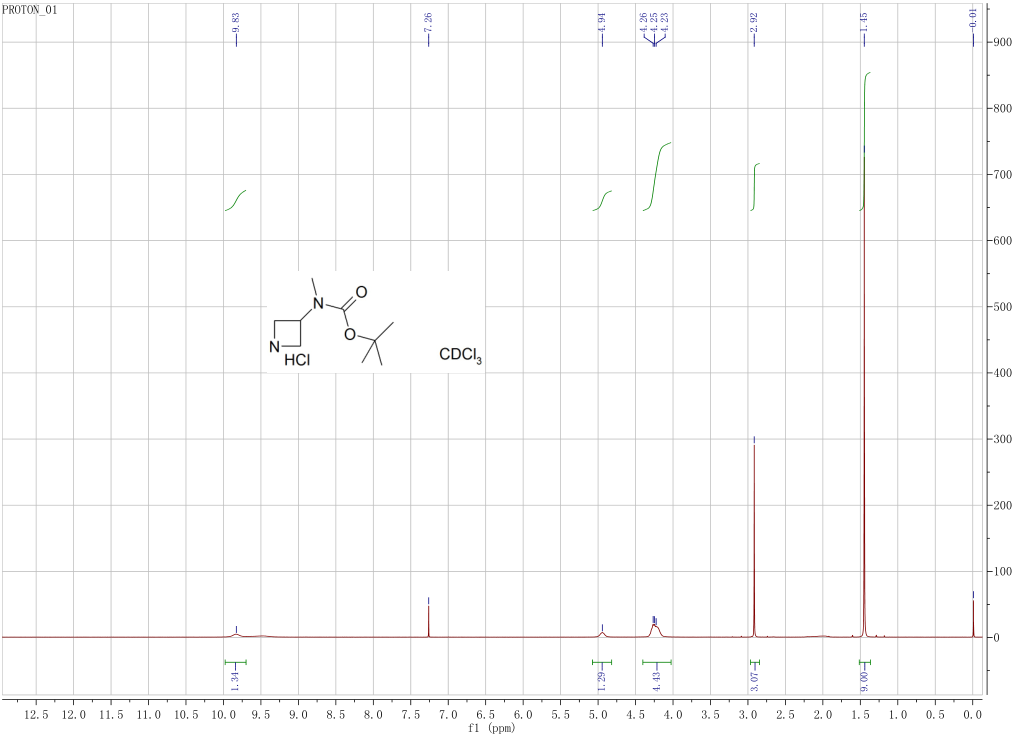
| 1H | chloroform-d1 | 300 | 1H NMR (300 MHz, CDCl3): δ 3.77 (t, J=6 Hz, 2H), 3.62 (t, J=6 Hz, 2H), 3.55 (m, 1H), 2.87 (s, 3H), 1.45 (s, 9H) | 3.77, 3.62, 3.55, 2.87, 1.45 | |
| 1H | chloroform-d1 | 1NMR (CDCI3): δ 1.45 (s, 9H), 2.87 (s, 3H), 3.62 (t, J = 6 Hz, 2H), 3.55 (m, 1 H), 3.77 (t, J = 6 Hz, 2H). | |||
| 1H | chloroform-d1 | 400 | 1R NMR (CDCl3) δ 1.45 (s, 9H), 2.9 (s, 3H), 3.65 (br, 2H), 3.75 (m, 2H), 4.6-5.0 (br, IH). |
| Description (Mass Spectrometry) | Comment (Mass Spectrometry) | Peak |
| DCI (Desorption chemical ionization) | Molecular peak | 187 m/z |
| DCI (Desorption chemical ionization) | Molecular peak |
Route of Synthesis (ROS)
| Conditions | Yield |
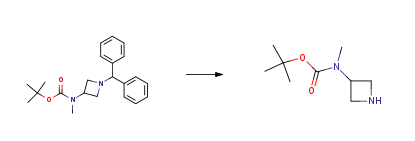
| With hydrogen; palladium 10% on activated carbon In methanol at 20℃; under 3040.2 Torr; Experimental Procedure 2-2.3 3) tert-butyl [1-(2-amino-6-chloropyrimidin-4-yl) azetidin-3-yl] methylcarbamate To a solution of tert-butyl [1- (diphenylmethyl) azetidin-3-yl] methylcarbamate (0.454 g) in MeOH (5 ml) was added 10% Pd/C (wet, H20 50.9%, 0.100 g), and the mixture was stirred under H2 atmosphere (4 atm) at room temperature overnight. The mixture was filtered through Celite pad, and the filtrate was concentrated in vacuo. The residue was dissolved in EtOH (2.0 ml), and N, N diisopropylethylamine (0.337 ml) and 2-amino-4,6- dichloropyrimidine (0.211 g) were added. The mixture was refluxed for 3 hours, cooled to room temperature, and the resulting solid was collected by filtration to give tert-butyl [1- (2-amino-6-chloropyrimidin-4-yl) azetidin-3-yl] methylcarbamate (0.231 g, 57% yield) as a solid. 1H NMR (500 MHz, CDC13) (51. 47 (s, 9H), 2.92 (s, 3H), 4.00-4. 08 (m, 2H), 4.21 (t, J= 8.8 Hz, 2H), 4.86 (s, 2H), 5.0 (br, 1H), 5.66 (s, 1H). | |
| With hydrogen; palladium 10% on activated carbon In methanol; water; ethyl acetate for 18h; Experimental Procedure 6.b (b) Title compoundA solution of the compound obtained above (6.18 g, 17.53 mmol) in 60 ml_ of MeOH and 15 ml_ of AcOEt was purged with argon. Pd/C (10%, 50% in water) (929 mg) was added and then, the solution was purged again with argon and stirred under H2 atmosphere for 18 hours. The reaction was filtered through Celite and the filtrate was washed with AcOEt and MeOH. The solvent was evaporated to dryness to afford 5.66 g of a mixture of the title compound together with one equivalent of diphenylmethane, which was further used as obtained.1H NMR (300 MHz, CD3O3) δ: 1.44 (s, 9H), 2.88 (s, 3H), 3.56 (m, 2H), 3.71 (m, 2H), 4.75 (m, 1 H). | |
| With hydrogen; palladium 10% on activated carbon In methanol; water; ethyl acetate for 18h; Experimental Procedure 2.b A solution of the compound obtained above (6.18 g, 17.53 mmol) in 60 mL of MeOH and 15 mL of EtOAc was purged with argon. Then Pd/C (10%, 50% in water) (929 mg) was added and the solution was purged again with argon and was stirred under H2 atmosphere for 18 hours. The reaction was filtered through CeI ite and the filtrate was washed with EtOAc and MeOH. The solvent was concentrated to dryness, to afford 5.66 g of a mixture of the title compound together with one equivalent of diphenylmethane, which was further used as obtained. 1H NMR (300 MHz, CD3OD) δ: 1.44 (s, 9H), 2.88 (s, 3H), 3.56 (m, 2H), 3.71 (m, 2H), 4.75 (m, 1 H). |
Safety and Hazards
| Precautionary Statement Codes | please visit ECHA C&L website |
| Source: European Chemicals Agency (ECHA) License Note: Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: “Source: European Chemicals Agency, http://echa.europa.eu/”. Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. License URL: https://echa.europa.eu/web/guest/legal-notice Record Name: (1-Cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluorophosphate URL: https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/213446 Description: The information provided here is aggregated from the “Notified classification and labelling” from ECHA’s C&L Inventory. Read more: https://echa.europa.eu/information-on-chemicals/cl-inventory-database |
Other Data
| Transportation | Under the room temperature and away from light |
| Storage | Under the room temperature and away from light |
| Market Price | USD |
| Use Pattern |
| 3-BOC-3-METHYLAMINOAZATIDINE 577777-20-9 Used as pharmaceutical intermediates. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |