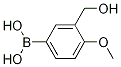
(3-(hydroxyMethyl)-4-Methoxyphenyl)boronic acid CAS#: 908142-03-0; ChemWhat Code: 1343361
Identification
Physical Data
| Appearance | Liquid |
Spectra
No data available
Route of Synthesis (ROS)
| Conditions | Yield |
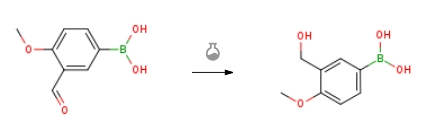
| With sodium tetrahydridoborate; ethanol In tetrahydrofuran at 0℃; for 2h; Inert atmosphere; Experimental Procedure 1 Synthesis of Intermediate Compound (a1) Under an argon gas flow, 3-formyl-4-methoxyphenylboronic acid of 10.00 g (55.6 mmol), tetrahydrofuran (THF, dehydrated) of 50 mL, and ethanol (dehydrated) of 50 mL were weighed into a reaction vessel of 300 mL and were stirred at 0° C. Sodium borohydride (NaBH4) of 1.36 g (36.0 mmol) was added thereto little by little. After stirring at 0° C. for 2 hours, the reaction was checked by TLC to know whether or not the raw materials had disappeared. When city water of 50 mL was added thereto to stop the reaction, a white precipitate was immediately formed.Then, the suspension was filtered under reduced pressure to remove the organic solvent. Hydrochloric acid having a concentration of 2 mol/L was added until the suspension became neutral. Then, the precipitate was collected by filtration. The filtered product was washed with ethyl acetate of 50 mL and was dried at 40° C. under reduced pressure to obtain an intermediate compound (a1) ((3-hydroxymethyl)-4-methoxy-phenyl) boronic acid) as the filtered product. The amount was 9.27 g (50.9 mmol), and the yield was 91.5%. | 91.5% |
| With sodium tetrahydridoborate; ethanol In tetrahydrofuran at 0℃; for 2h; Inert atmosphere; Experimental Procedure First, in an argon gas flow, 10.00 g (55.6 mmol) of 3-formyl-4-methoxyphenylboronic acid, 50 mL of tetrahydrofuran (THF, dehydration), and 50 mL of ethanol (dehydration) were measured and put in a 300 milliliter-reactor vessel, and the resultant was stirred at a temperature of 0 degrees Celsius. 1.36 g (36.0 mmol) of sodium borohydride (NaBH4) were added to the resultant by a small amount each. After stirring at a temperature of 0 degrees Celsius for two hours, the reaction check was performed with TLC, and disappearance of the raw materials was confirmed. Immediately after 50 mL of city water were added to the resultant to stop the reaction, white precipitate was generated promptly. Subsequently, the suspension was subjected to filtration under a low pressure, and an organic solvent was removed. Hydrochloric acid having a concentration of 2 mol/L was added until the suspension became neutral. After that, the precipitate was recovered through filtration. The recovered matters through filtration were washed with 50 mL of ethyl acetate, and were dried under a low pressure at a temperature of 40 degrees Celsius. Thus, as the recovered matters, an intermediate compound (iii-1) ((3-hydroxymethyl)-4-methoxy-phenyl) boronic acid) was obtained. The yield amount was 9.27 g (50.9 mmol), and the yield was 91.5%. | 91.5% |
| With sodium tetrahydridoborate; ethanol In tetrahydrofuran at 0℃; for 2h; Inert atmosphere; Experimental Procedure First, in an argon gas flow, 10.00 g (55.6 mmol) of 3-formyl-4-methoxyphenylboronic acid, 50 mL of tetrahydrofuran (THF, dehydration), and 50 mL of ethanol (dehydration) were measured and put in a 300 milliliter-reactor vessel, and the resultant was stirred at a temperature of 0 degrees Celsius. 1.36 g (36.0 mmol) of sodium borohydride (NaBH4) were added to the resultant by a small amount each. After stirring at a temperature of 0 degrees Celsius for two hours, the reaction check was performed with TLC, and disappearance of the raw materials was confirmed. Immediately after 50 mL of city water were added to the resultant to stop the reaction, white precipitate was generated promptly. Subsequently, the suspension was subjected to filtration under a low pressure, and an organic solvent was removed. Hydrochloric acid having a concentration of 2 mol/L was added until the suspension became neutral. After that, the precipitate was recovered through filtration. The recovered matters through filtration were washed with 50 mL of ethyl acetate, and were dried under a low pressure at a temperature of 40 degrees Celsius. Thus, as the recovered matters, an intermediate compound (iii-1) ((3-hydroxymethyl)-4-methoxy-phenyl) boronic acid) was obtained. The yield amount was 9.27 g (50.9 mmol), and the yield was 91.5%. | 91.5% |
| With sodium tetrahydridoborate In tetrahydrofuran; ethanol at 0 – 20℃; for 2h; | |
| With sodium tetrahydridoborate Experimental Procedure (Production of Main Agent 3) First, a main agent 3 (a compound represented by Formula (iii)) described later was produced in accordance with the following method. First, in an argon gas flow, 10.00 g (55.6 mmol) of 3-formyl-4-methoxyphenylboronic acid, 50 mL of tetrahydrofuran (THF, dehydration), and 50 mL of ethanol (dehydration) were measured and put in a 300 milliliter-reactor vessel, and the resultant was stirred at a temperature of 0 degrees Celsius. 1.36 g (36.0 mmol) of sodium borohydride (NaBH4) were added to the resultant by a small amount each. After stirring at a temperature of 0 degrees Celsius for two hours, the reaction check was performed with TLC, and disappearance of the raw materials was confirmed. Immediately after 50 mL of city water were added to the resultant to stop the reaction, white precipitate was generated promptly. Subsequently, the suspension was subjected to filtration under a low pressure, and an organic solvent was removed. Hydrochloric acid having a concentration of 2 mol/L was added until the suspension became neutral. After that, the precipitate was recovered through filtration. The recovered matters through filtration were washed with 50 mL of ethyl acetate, and were dried under a low pressure at a temperature of 40 degrees Celsius. Thus, as the recovered matters, an intermediate compound (iii-1) ((3-hydroxymethyl)-4-methoxy-phenyl) boronic acid) was obtained. The yield amount was 9.27 g (50.9 mmol), and the yield was 91.5%. |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] |
| Precautionary Statement Codes | P264, P264+P265, P280, P302+P352, P305+P351+P338, P321, P332+P317, P337+P317, and P362+P364 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| HS Code | |
| Storage | Store at 2~8° for long time. |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 181.984 |
| logP | 0.151 |
| HBA | 3 |
| HBD | 3 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 69.92 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Use Pattern |
| (3-(Hydroxymethyl)-4-methoxyphenyl)boronic acid is a boronic acid derivative with several important applications, primarily in the fields of organic synthesis and pharmaceuticals. It can serve as an intermediate in the synthesis of other complex organic molecules, particularly in the synthesis of pharmaceuticals and natural products. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Limited | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |