3-(N-Tosyl-L-alaninyloxy)-5-phenylpyrrole CAS#: 99740-00-8; ChemWhat Code: 1338959
Identification
| Product Name | 3-(N-Tosyl-L-alaninyloxy)-5-phenylpyrrole |
| IUPAC Name | (5-phenyl-1H-pyrrol-3-yl) (2S)-2-[(4-methylphenyl)sulfonylamino]propanoate |
| Molecular Structure | 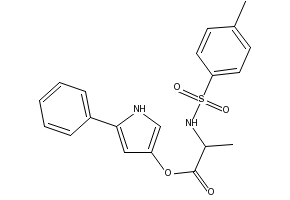 |
| CAS Registry Number | 99740-00-8 |
| EINECS Number | No data available |
| MDL Number | MFCD08704359 |
| Beilstein Registry Number | No data available |
| Synonyms | 3-Pyridinamin;3-Pyridinamine;3-Pyridinamine;pyridin-3-amine;T6NJ CZ;3- Aminopyridine;3-Amino-pyridine;3-pyridylamine;Amino-3 pyridine;m-Aminopyridine;MS/MS-1064463;Pyridin-3-ylamine;Pyridine, 3-amino-;β-Aminopyridine 462-08-8 |
| Molecular Formula | C20H20N2O4S |
| Molecular Weight | 384.450 |
| InChI | InChI=1S/C20H20N2O4S/c1-14-8-10-18(11-9-14)27(24,25)22-15(2)20(23)26-17-12-19(21-13-17)16-6-4-3-5-7-16/h3-13,15,21-22H,1-2H3/t15-/m0/s1 |
| InChI Key | VZALJYJCTCKOHO-HNNXBMFYSA-N |
| Canonical SMILES | Cc1ccc(S(=O)(=O)NC@@HC(=O)Oc2c[nH]c(-c3ccccc3)c2)cc1 |
| Patent Information |
| No data available |
Physical Data
| Appearance | White to off-white solid |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
Spectra
| No data available |
Route of Synthesis (ROS)
| Conditions | Yield |
| Experimental Procedure 3-(N-tosyl-L-alaninyloxy)-5-phenylpyrrole (4) 3-(N-tosyl-L-alaninyloxy)-5-phenylpyrrole (4) A solution of anhydrous tetrahydrofuran (THF, 450 mL), pyridine (43.8 mL; 0.542 mol; 1.2 eq) and trifluoroacetic acid (85.0 mL; 1.10 mol; 2.4 eq), maintained at 0° C. under an inert gas atmosphere, was treated in one portion with 3-hydroxy-5-phenylpyrrole (3) (71.5 g; 0.45 mol; 1.0 eq) followed immediately by the dropwise addition, over 5-10 minutes of a solution of freshly prepared N-tosyl-L-alaninyl chloride (141.0 g; 0.54 mol; 1.2 eq) in anhydrous THF (450 mL). The resulting mixture was stirred for 15 minutes at 0° C. The reaction was then quenched by addition of a solution of 1.0M aqueous citric acid (315 mL) and EtOAc (1.35 L). After brief mixing the phases were separated and the organic layer washed with a solution of aqueous NaCl (360 mL; 0.18 g NaCl per mL of water). The organic layer was next extracted twice with a solution of 5% aqueous NaHCO3 (1.35 L each), and then washed with another portion of aqueous NaCl (360 mL; 0.18 g NaCl per mL of water). The reddish brown organic layer was stirred at ambient temperature for 15 minutes with MgSO4 (101 g) and Darco-G60 (143 g), then filtered through Celite and evaporated to dryness in vacuo from a 37° bath to give (4) as a pinkish-white solid. | 99% |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 384.456 |
| logP | 4.178 |
| HBA | 5 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 96.64 |
| Rotatable Bond (RotB) | 7 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Mainly used for esterase reagents and white blood cell testing. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |