3-Phenylpyridine CAS#: 1008-88-4; ChemWhat Code: 13581
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN112707857 | Method for preparing piperidine compound by reducing pyridine compound through hydrogen transfer | 2021 |
| EP1473286 | PROCESS FOR PRODUCING BIARYL COMPOUND | 2004 |
| US4386209 | Chichibabin reaction | 1983 |
Physical Data
| Appearance | Light yellow liquid |
| Solubility | Soluble in chloroform, dichloromethane and ethyl acetate. |
| Flash Point | No data available |
| Refractive index | n20/D 1.616(lit.) |
| Sensitivity | No data available |
| Melting Point, °C |
| 162-163 |
| 115 – 116 |
| 176 |
| 63 – 64 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 269 | 760 |
| 100 – 110 | 5 |
| 74 – 80 | 1 |
| 85 | 0.01 |
| 130 – 140 | 0.2 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.518 | -173.16 |
| 1.24 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Stability constant of the complex with … | CCl4 | 10 – 40 | methyltrioxorhenium(VII) |
| Enthalpy of association | CCl4 | 25 | methyltrioxorhenium(VII) |
| NMR spectrum of the complex | CD3CN | 22.9 | methyltrioxorhenium |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 126 |
| DEPT (Distorsionless Enhancement by Polarisation Transfer), Chemical shifts, Spectrum | 13C | chloroform-d1 | 100.4 |
| COSY (Correlation Spectroscopy), Spectrum | 1H, 1H | chloroform-d1 | 400 |
| HSQC (Heteronuclear Single Quantum Coherence), Spectrum | 1H, 13C | chloroform-d1 | |
| HMBC (Heteronuclear Multiple Bond Coherence), Spectrum | 1H, 13C | chloroform-d1 | |
| Spectrum | 13C | chloroform-d1 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| ATR (attenuated total reflectance), Bands | |
| Bands | potassium bromide |
| ATR (attenuated total reflectance), Bands, Spectrum | neat (no solvent, solid phase) |
| Intensity of IR bands, ATR (attenuated total reflectance), Bands | neat liquid |
| Description (Mass Spectrometry) |
| electron impact (EI), gas chromatography mass spectrometry (GCMS), spectrum |
| electron impact (EI), spectrum |
| gas chromatography mass spectrometry (GCMS), electron impact (EI), time-of-flight mass spectra (TOFMS), spectrum |
| gas chromatography mass spectrometry (GCMS), spectrum |
| gas chromatography mass spectrometry (GCMS), electron impact (EI), spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | 204, 245, 275 | 4.44, 4.23, 3.9 | ||
| Spectrum | acetonitrile | |||
| Spectrum | acetonitrile | |||
| Absorption maxima | ||||
| Spectrum | ethanol | 205 – 300 nm |
| Description (Raman Spectroscopy) | Solvent (Raman Spectroscopy) |
| Spectrum | neat (no solvent) |
| Spectrum | CHCl3 |
| Spectrum | CCl4 |
| Bands | CCl4 |
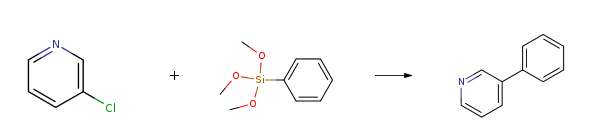
Route of Synthesis (ROS)
| Conditions | Yield |
| With sodium hydroxide; tetrabutylammomium bromide; 4,4′-dichlorobenzophenone oxime-derived palladacycle at 120℃; under 7500.6 Torr; for 0.166667h; Hiyama coupling; microwave irradiation; | 92% |
| With 3-tert-butyl-1-(2-(dicyclohexylphosphino)phenyl)-5-methyl-1H-pyrazole; tetrabutyl ammonium fluoride; palladium diacetate In 1,4-dioxane at 100℃; for 4h; Reagent/catalyst; Hiyama Coupling; Inert atmosphere; | 88% |
| With 3-tert-butyl-1-(2-(dicyclohexylphosphino)phenyl)-5-methyl-1H-pyrazole; tetrabutyl ammonium fluoride; palladium diacetate In 1,4-dioxane at 100℃; for 4h; Reagent/catalyst; Hiyama Coupling; Inert atmosphere; | 88% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (99.5%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 6 months |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 155.199 |
| logP | 2.71 |
| HBA | 1 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 12.89 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Use Pattern |
| 3-Phenylpyridine CAS#: 1008-88-4 used as intermediates. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |