3,4-Epoxycyclohexylmethyl 3,4-epoxycyclohexanecarboxylate CAS#: 2386-87-0; ChemWhat Code: 211630
Identification
| Product Name | 3,4-epoxycyclohexylmethyl 3,4-epoxycyclohexanecarboxylate |
| IUPAC Name | 7-oxabicyclo[4.1.0]heptan-3-ylmethyl 7-oxabicyclo[4.1.0]heptane-3-carboxylate |
| Molecular Structure | 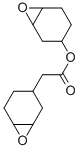 |
| CAS Registry Number | 2386-87-0 |
| Synonyms | 3,4-epoxycyclohexylmethyl 3,4-epoxycyclohexanecarboxylate, (3,4-epoxycyclohexane)methyl 3,4-epoxycyclohexylcarboxylate, 3,4-epoxycyclohexylmethyl-3,4-epoxycyclohexylcarboxylate, Cyracure UVR-6105, UVR 6110, 7-oxabicyclo[4.1.0]hept-3-ylmethyl 7-oxabicyclo[4.1.0]heptane-3-carboxylate, 3,4-epoxycyclohexanecarboxylicacid-3′,4’epoxycyclohexylmethylester |
| Molecular Formula | C14H20O4 |
| Molecular Weight | 252.31 |
| InChI | InChI=1S/C14H20O4/c15-14(9-2-4-11-13(6-9)18-11)16-7-8-1-3-10-12(5-8)17-10/h8-13H,1-7H2 |
| InChI Key | YXALYBMHAYZKAP-UHFFFAOYSA-N |
| Canonical SMILES | C1CC2C(O2)CC1COC(=O)C3CCC4C(C3)O4 |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| TW2017/6269 | CURABLE COMPOSITIONS CONTAINING BENZOXAZINE EPOXY BLEND AND USE THEREOF | 2017 |
| WO2006/39440 | POLY(ARYLENE ETHER) COMPOSITION | 2006 |
| US2007/167645 | Radiation-cured substances | 2007 |
| US6359147 | Reactions catalyzed by chromium (III) carboxylates | 2002 |
Physical Data
| Appearance | Viscous liquid |
| Melting Point | −37 °C (lit.) |
| Flash Point | 118 °C |
| Density | 1.17 g/mL at 25 °C(lit.) |
| refractive index | n20/D 1.498(lit.) |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Stability constant of the complex with … | CCl4 | 19.9 | iodine |
| UV/VIS spectrum of the complex | CCl4 | 19.9 | iodine |
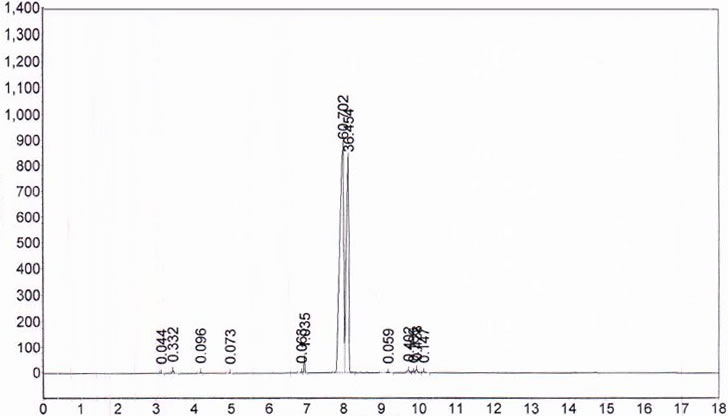
Spectra
Route of Synthesis (ROS)
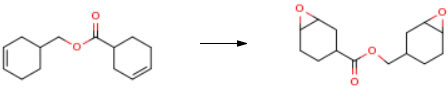
| Conditions | Yield |
| With phosphotungstic acid; trioctylmethylammonium acetate; phosphoric acid; dihydrogen peroxide; sodium carbonate In water; toluene at 50℃ for 15h Inert atmosphere Experimental Procedure mixer,Reflux condenser,In a flask equipped with a stirrer,12 parts of water was added while purging with nitrogen,0.38 parts of 12-tungstophosphoric acid,0.56 parts of phosphoric acid,Sodium carbonate was added,The pH was adjusted to 5.0.Further, 0.6 part of trioctylmethylammonium acetate (xylene solution of trioctylmethylammonium acetate manufactured by Lion Akzo) was added,After refining the tungstic acid type catalyst,35 parts of toluene was added and dissolved,As a two-layer system solution,The mixture was stirred vigorously at room temperature for 1 hour.Here, equation (1).And 22 parts of the compound represented by the formulaThe solution was heated to 50 ° C.,While stirring,24.8 parts of 30% by weight hydrogen peroxide water was added,The mixture was further stirred at 50 ° C. for 15 hours as a postreaction.After cooling to room temperature,1 part of 30% by weight sodium hydroxide aqueous solution,10 parts of 20% by weight aqueous sodium thiosulfate solution was added and stirred for 1 hour,After 15 parts of toluene was further added,It was left standing.after that,The organic layer separated into two layers was taken out.To the obtained organic layer, 8.8 parts of Hokuetsu HS (phenol · formaldehyde resin beads manufactured by Ajinomoto Fine-Techno Co., Ltd.) which had been washed with methanol in advance was added,The mixture was stirred at room temperature for 2 hours and filtered under reduced pressure.The resulting solution was washed three times with 30 parts of water,Using a rotary evaporator,By distilling off the organic solvent,20 parts of a desired epoxy compound (EP1) was obtained.The obtained epoxy compound was pale yellow,Epoxy equivalent is 130 g / eq. , And the viscosity was 241 mPa · s.The amount of remaining quaternary ammonium salt (gas chromatography) was 50 ppm,The residual tungsten content (ashing method) was 1 ppm.The obtained epoxy compound contained 6% monoepoxy compound,93% diepoxy compound,It is 1% water adduct,Main purity measurement by GPC showed 99%. | 94% |
| With 1H-imidazole; tristetrahexylammonium tetrakis(diperoxotungsto) phosphate; dihydrogen peroxide In water; acetonitrile at 49.84℃ under 760.051 Torr for 24h Reagent/catalyst Inert atmosphere | 82% |
| With dihydrogen peroxide; acetonitrile; triethylamine In water at 60℃ for 10h Conversion of starting material Experimental Procedure Examples 2 to 5The reactions were carried out in the same manner as in Example 1 by changing the reaction conditions (liquid composition), and the results are shown in Table 1 together with the results of Example 1. Incidentally, in Example 5, a substrate different from that of Example 1 was used, but the concentration of the substrate was the same as in Example 1, i.e., 0.448 mol. In Example 4 using benzonitrile as the nitrile compound, the conversion ratio was higher compared with other Examples using acetonitrile and the reactivity was good. In Example 5, the conversion ratio was lower compared with Example 1 employing the same synthesis conditions, and the product was monoepoxide only. It was revealed that the reaction selectively occurs with the double bond of the cyclohexene skeleton. | 46% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H317: May cause an allergic skin reaction [Warning Sensitization, Skin] H412: Harmful to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P272, P273, P280, P302+P352, P321, P333+P313, P363, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page. |
Other Data
| Transportation | Not dangerous goods |
| HS Code | 291899 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD 24000/MT |
| Use Pattern |
| 3,4-Epoxycyclohexylmethyl 3,4-epoxycyclohexanecarboxylate CAS#: 2386-87-0 is used as dental composition for making dental prostheses and for dental restoration |
| Component of anti-fouling varnish composition of invention |
| in epoxy resin compositions |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Lambson Limited(UK) | http://www.lambson.com/ |
| Daicel Corporation.(Japan) | https://www.daicel.com/ |
| Warshel Chemical Ltd | https://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |