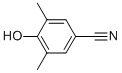
3,5-Dimethyl-4-hydroxybenzonitrile CAS#: 4198-90-7; ChemWhat Code: 62810
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2023/176725 | ALLYL ETHER COMPOUND AND METHOD FOR PRODUCING SAME, CURABLE RESIN COMPOSITION, VARNISH, PREPREG, CURED PRODUCT, POLYPHENYLENE ETHER RESIN CURING AGENT, AND CRYSTALS AND METHOD FOR PRODUCING SAME | 2023 |
| CN104530078 | A thieno [3, 2 – d] pyrimidine derivative and its preparation method and application | 2017 |
| US2013/23563 | AMIDINOANILINE DERIVATIVE | 2013 |
| EP2213650 | SUBSTITUTED DIPHENYLAMINES AS INHIBITORS OF REVERSE TRANSCRIPTASE, PROCESS OF PREPARING THEM AND USE THEREOF | 2010 |
Physical Data
| Appearance | White solid |
| Melting Point, °C | Solvent (Melting Point) |
| 72 – 74 | |
| 165 – 166 | |
| 96 – 98 | |
| 125 | aq. ethanol |
| 124 – 125 | |
| 124 – 124.4 | petroleum ether |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | 400 |
Route of Synthesis (ROS)
| Conditions | Yield |
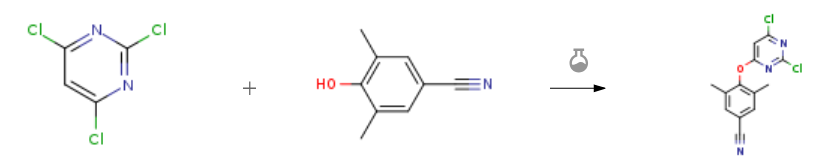
| With N-ethyl-N,N-diisopropylamine In 1,4-dioxane at 70℃; for 2h; Experimental Procedure A mixture of 2,4,6-trimethylpyrimidine II(110 mmol, 20.0 g) and 3,5-dimethyl-4-hydroxybenzonitrile III (110mmol, 16.2 g) Was reacted with N, N-diisopropylethylamine (DIEA) (132 mmol, 17. Og) in 100 mL of 1,4-dioxane at 70 ° C for 2 h, When the reaction solution was cooled to about 10 ° C, A 200 mL aqueous solution was slowly added to the reaction solution and stirred for 30 min, filtered and dried in vacuo to give 29.8 g of intermediate IV in a yield of 92.5% | 92.5% |
| With N-ethyl-N,N-diisopropylamine In 1,4-dioxane at 70℃; for 2h; Experimental Procedure 4.1.4 4-((2,6-Dichloropyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (7) 2,4,6-trichloropyrimidine (6, 11mmol, 2.0g), DIEPA (13mmol, 1.7g) and 4-hydroxy-3,5-dimethylbenzonitrile (11mmol, 1.6g) were dissolved in 10mL 1,4-dioxane and the mixed solution were heated at 70°C for 2h. After the reaction mixture was brought to room temperature, 50mL cold water was poured into the mixture and stirred for another 30min, filtrated. The wet cake was dried at 55-60°C under vacuum to give the intermediate 7 as white solid with a yield of 92%, mp: 207-209°C. 1H NMR (400MHz, DMSO-d6, ppm) δ: 7.76 (s, 2H, C3,C5-Ph-H), 7.64 (s, 1H, pyrimidine-H), 2.12 (s, 6H). ESI-MS: m/z 294.2 [M+1]+. C13H9Cl2N3O (293.01). | 92% |
| With N-ethyl-N,N-diisopropylamine In 1,4-dioxane at 70℃; for 2h; Experimental Procedure Weigh 2,4,6-trichloropyrimidine (2.0g, 10.9mmol), 4-hydroxy-3,5-dimethylbenzonitrile (1.6g, 10.9mmol) and N,-diisopropylethylamine (3.6ml, 21.8mmol) in 25mL of 1,4-dioxane solution, stirred at 70 °C for 2h. After the reaction was detected by TLC, after the reaction solution was cooled, 100 mL of water was slowly added thereto, stirring was continued for 30 min, filtration was performed, and the vacuum drying oven was used for drying. A white solid was obtained as the compound 4-((2,6-dichloropyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (2) in a yield of 91.8%. | 91.8% |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H300 (86.67%): Fatal if swallowed [Danger Acute toxicity, oral] H302 (11.11%): Harmful if swallowed [Warning Acute toxicity, oral] H315 (13.33%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (11.11%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P264, P264+P265, P270, P271, P280, P301+P316, P301+P317, P302+P352, P304+P340, P305+P351+P338, P319, P321, P330, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Under the room temperature and away from light | |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 94147.177 |
| logP | 1.791 |
| HBA | 1 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 44.02 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Use Pattern |
| 3,5-Dimethyl-4-hydroxybenzonitrile CAS 4198-90-7 used as an intermediate in the synthesis of various drugs. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |