(3aR,4S,6R,6aS)-6-Aminotetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-ol CAS#: 155899-66-4; ChemWhat Code: 410972
Identification
| Product Name | (3aR,4S,6R,6aS)-6-Aminotetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-ol |
| IUPAC Name | (3aR,4S,6R,6aS)-6-amino-2,2-dimethyl-4,5,6,6a-tetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-ol |
| Molecular Structure | 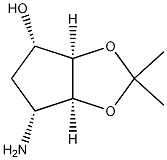 |
| CAS Registry Number | 155899-66-4 |
| MDL Number | MFCD22690255 |
| Synonyms | (3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-ol, [3aR-(3aα,4α,6α,6aα)]-6-amino-tetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-dioxo14-ol, (+/-)-(3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-ol, (3aR,4S,6R,6aS)-6-amino-2,2-dimethyl-tetrahydro-3aH-cyclopenta[d][ 1,3]dioxol-4-ol, (3aR,4S,6R,6aS)-6-amino-2,2-dimethyl-tetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-ol, (3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-ol, (3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetraydro-3aH-cyclopenta[d][1,3]dioxol-4-ol |
| Molecular Formula | C8H15NO3 |
| Molecular Weight | 173.21 |
| InChI | InChI=1S/C8H15NO3/c1-8(2)11-6-4(9)3-5(10)7(6)12-8/h4-7,10H,3,9H2,1-2H3 |
| InChI Key | AXPYGRDXRLICKY-JRTVQGFMSA-N |
| Canonical SMILES | CC1(OC2C(CC(C2O1)O)N)C |
| Isomeric SMILES | CC1(O[C@H]2[C@@H](C[C@@H]([C@H]2O1)O)N)C |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| EP2666771 | Synthesis of Aminocyclopentanetriol Derivatives | 2013 |
| US2014/206867 | Process for Preparing Cyclopentylamine Derivatives and Intermediates Thereof | 2014 |
Physical Data
| Appearance | Off-white to white powder |
| Boiling Point | 288 °C |
| Flash Point | 128 °C |
| Density | 1.170 g/cm3 at 20°C (68°F) |
| Melting Point, °C | Solvent (Melting Point) |
| 85.5 – 86.6 | |
| 85 – 88 | CH2Cl2, hexane |
| 125.5 – 127 | diethyl ether |
| Type (Optical Rotatory Power) | Concentration (Optical Rotatory Power) | Solvent (Optical Rotatory Power) | Optical Rotatory Power, deg | Wavelength (Optical Rotatory Power), nm | Temperature (Optical Rotatory Power), °C |
| [alpha] | 0.3g/100ml | chloroform | -50.9 | 589 | |
| [alpha] | 0.33g/100ml | CHCl3 | 23.2 | 589 | 25 |
| [alpha] | 0.3g/100ml | CHCl3 | 22 | 589 | 25 |
| [alpha] | 0.33g/100ml | CHCl3 | -51.5 | 589 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Chemical shifts | 13C | CDCl3 | 75 |
Route of Synthesis (ROS)
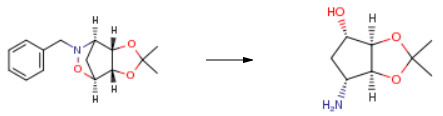
| Conditions | Yield |
| With palladium 10% on activated carbon; hydrogen In methanol at 30℃; under 2250.23 Torr; for 18h; Experimental Procedure A mixture of G’ (60.0 g), 10 percent Pd/C (6.0 g) and methanol (0.60 L) was stirred under hydrogen (3 bar) at 30 °C for 18 hours. The palladium on charcoal was removed by filtration ant the solvent was removed to give H (40 g, 100 percent). 1 H NMR (CDCI3): δ 1 .29 (s, 3H), 1 .40 (s, 3H), 1 .58 (m, 1 H), 1 .65 (m, 1 H), 2.09 (m, 1 H), 3.60 (m, 1 H), 4.09 (m, 1 H), 4.41 (m, 1 H), 4.69 (m, 1 H) ppm. | 100% |
| With hydrogen; ammonium formate; palladium on activated charcoal In methanol at 20℃; for 2h; Experimental Procedure A mixture of VII’ (60.0 g), 10 percent Pd/C (6.0 g) and methanol (0.60 L) was stirred under hydrogen (3 bar) at 30 °C for 18 hours. The palladium on charcoal was removed by filtration ant the solvent was removed to give IX (40 g, 100 percent). 1H NMR (CDCl3): δ 1.29 (s, 3H), 1.40 (s, 3H), 1.58 (m, 1H), 1.65 (m, 1H), 2.09 (m, 1H), 3.60 (m, 1H), 4.09 (m, 1H), 4.41 (m, 1H), 4.69 (m, 1H) ppm. | 98% |
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; for 14h; Experimental Procedure A solution of 17 (100 mg, 0.38 mmol, 1 eq) in methanol (2 mL) was hydrogenated for 14 h at r.t. over 10percent palladium on carbon (15 mg). The reaction mixture was then filtered, and the collected solids were washed with methanol (1 mL). The combined filtrate was concentrated under reduced pressure to give the title product as a colorless oil, which could be recrystallized by PE to give a white solid (60 mg, 92.3percent yield). 1H NMR (500 MHz, CDCl3) δ 1.29 (3 H, s), 1.40 (3 H, s), 1.61~1.66 (1 H, d), 2.06~2.13 (1 H, m), 2.63 (2 H, s), 3.60~3.61 (1 H, d), 4.09~4.10 (1 H, d), 4.40~4.42 (1 H, d), 4.68~4.70 (1 H, d). EI-MS m/z 174.1 (M+H)+. | 92.3% |
| Multi-step reaction with 2 steps 1: 78 percent / Zn, AcOH / diethyl ether / 48 h / Ambient temperature 2: 75 percent / ammonium formate / Pd/C / methanol / 1 h / Heating | |
| Multi-step reaction with 2 steps 1.1: zinc; acetic acid / diethyl ether / 72 h / 0 – 25 °C 1.2: pH 7.2 2.1: hydrogen; palladium 10% on activated carbon / methanol / 5 h / 30 °C / 2250.23 Torr | |
| Multi-step reaction with 2 steps 1: zinc; acetic acid / diethyl ether / 72.48 h / 0 – 25 °C 2: palladium 10% on activated carbon; hydrogen / methanol / 5 h / 30 °C / 2250.23 Torr |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H315: Causes skin irritation [Warning Skin corrosion/irritation] H318: Causes serious eye damage [Danger Serious eye damage/eye irritation] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P264, P280, P302+P352, P305+P351+P338, P310, P321, P332+P313, P337+P313, and P362 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | |
| Market Price | USD 1140/kg |
| Use Pattern |
| (3aR,4S,6R,6aS)-6-Aminotetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-ol CAS#: 155899-66-4 is used as the Pharmaceutical Intermediates |
| bacterial infections |
| Preservative agent for compositions for udder hygiene |
| Preservative agent for compositions for teat hygiene |
| Preservative agent for compositions for treatment of mastitis |
| (3aR,4S,6R,6aS)-6-Aminotetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-ol CAS#: 155899-66-4 is used in surface coating systems |
| Fertiliser for agricultural compositions |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | https://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

