4-Hydroxyphthalic acid CAS#: 610-35-5; ChemWhat Code: 58552
Identification
| Product Name | 4-Hydroxyphthalic acid |
| IUPAC Name | 4-hydroxyphthalic acid |
| Molecular Structure | 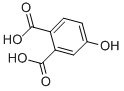 |
| CAS Registry Number | 610-35-5 |
| EINECS Number | 210-221-6 |
| MDL Number | MFCD00013984 |
| Synonyms | 4-hydroxyphthalic acid, 4-Hydroxyphthalic acid, (4-hydroxy)phthalic acid, 4′-hydroxyphthalic acid, 4-hydroxy-phthalic acid, 3-hydroxyphthalic acid, 4-hydroxyphtalic acid, CAS Number 610-35-5, CAS NO 610-35-5 |
| Molecular Formula | C8H6O5 |
| Molecular Weight | 182.133 |
| InChI | InChI=1S/C8H6O5/c9-4-1-2-5(7(10)11)6(3-4)8(12)13/h1-3,9H,(H,10,11)(H,12,13) |
| InChI Key | MWRVRCAFWBBXTL-UHFFFAOYSA-N |
| Canonical SMILES | C1=CC(=C(C=C1O)C(=O)O)C(=O)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2015/56305 | DITHIOL MUCOLYTIC AGENTS | 2015 |
| US8653160 | Inclusion complex containing epoxy resin composition for semiconductor encapsulation | 2014 |
| US2013/90404 | Dental materials with improved hydrolysis stability based on phthalic acid monomers | 2013 |
| US5516672 | Stabilized peroxidase compositions and antibody compositions | 1996 |
Physical Data
| Appearance | White to off-white powder |
| Water Solubility | Slightly miscible with water |
| Melting Point, °C | Solvent (Melting Point) |
| 202 – 203 | ethyl acetate |
| 196 – 199 | |
| 204 | H2O |
| Decomposition, °C | Solvent for Crystallization (Decomposition) | Amount (Decomposition), mol |
| 190 | ||
| 111 | water | 0.7 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 400 |
| Chemical shifts | 13C | dimethylsulfoxide-d6 | 100 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | potassium bromide |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Absorption maxima | H2O, acetonitrile | 245, 280 |
Route of Synthesis (ROS)

| Conditions | Yield |
| Stage #1: 4-sulfophthalic acid With sodium hydroxide In water at 180 – 200℃; for 2h; Stage #2: With hydrogenchloride In water pH=1; Cooling with ice; Experimental Procedure 4-hydroxyphthalic Acid Sodium hydroxide (251.9 g, 6.30 mol) was added in portions to 4-sulphophthalic acid (50percent in water, 258.4 g, 0.525 mol) in a steel vessel accompanied by stirring. After the addition of approx. one third NaOH the increasingly more viscous mixture was heated to 180° C. Once the addition had ended the mixture was stirred for a further 2 h at 200° C. During cooling, the residue was dissolved in water (1000 ml). Conc. hydrochloric acid (620 ml, pH=1) was added accompanied by ice cooling. The solution was extracted with ethyl acetate (5*400 ml). The combined organic phases were dried over Na2SO4, filtered and concentrated on the rotary evaporator. The slightly yellow solid was recrystallized from ethyl acetate. 68.64 g (0.377 mol; 72percent yield) of a white solid was obtained. 1H-NMR (DMSO-d6, 400 MHz): δ=6.90-6.93 (m, 2H), 7.69 (dd, 1H; J=2.4 Hz, 6.6 Hz), 10.41 (br s, 1H), 12.87 (br s, 2H). 13C-NMR (DMSO-d6, 100 MHz): δ=114.2, 116.2, 120.7, 131.5, 137.3, 160.1, 167.4, 169.5. | 72% |
| With water; sodium hydroxide at 180 – 200℃; for 2h; Experimental Procedure Sodium hydroxide (251.9 g, 6.30 mol) was added in portions to 4-sulphophthalic acid (50percent in water, 258.4 g, 0.525 mol) in a steel vessel accompanied by stirring. After the addition of approx. one third NaOH the increasingly more viscous mixture was heated to 180° C. Once the addition had ended the mixture was stirred for a further 2 h at 200° C. During cooling, the residue was dissolved in water (1000 ml). Conc. hydrochloric acid (620 ml, pH=1) was added accompanied by ice cooling. The solution was extracted with ethyl acetate (5*400 ml). The combined organic phases were dried over Na2SO4, filtered and concentrated on the rotary evaporator. The slightly yellow solid was recrystallized from ethyl acetate. 68.64 g (0.377 mol; 72percent yield) of a white solid was obtained. 1H-NMR (DMSO-d6, 400 MHz): δ=6.90-6.93 (m, 2H), 7.69 (dd, 1H; J=2.4 Hz, 6.6 Hz), 10.41 (br s, 1H), 12.87 (br s, 2H). 13C-NMR (DMSO-d6, 100 MHz): δ=114.2, 116.2, 120.7, 131.5, 137.3, 160.1, 167.4, 169.5. | 72% |
| Stage #1: 4-sulfophthalic acid With sodium hydroxide; water at 200 – 210℃; for 2.5h; Heating / reflux; Stage #2: With hydrogenchloride; water at 0 – 50℃; | 71.5% |
| With sodium hydroxide In water at 180℃; for 1h; | 23% |
| With sodium hydroxide at 175℃; |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315: Causes skin irritation [Warning Skin corrosion/irritation] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P264, P280, P302+P352, P305+P351+P338, P321, P332+P313, P337+P313, and P362 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | |
| Market Price |
| Use Pattern |
| 4-hydroxyphthalic acid has a wide range of applications in the synthesis of pharmaceutical intermediates |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

