5-Amino-4-imidazolecarboxamide CAS#: 360-97-4; ChemWhat Code: 26003
Identification
| Product Name | 5-Amino-4-imidazolecarboxamide |
| IUPAC Name | 4-amino-1H-imidazole-5-carboxamide |
| Molecular Structure | 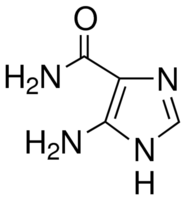 |
| CAS Registry Number | 360-97-4 |
| EINECS Number | 206-641-4 |
| MDL Number | MFCD02181040 |
| Beilstein Registry Number | No data available |
| Synonyms | 5-Aminoimidazole-4-carboxamide, 5-aminoimidazole-4-carboxamide |
| Molecular Formula | C4H6N4O |
| Molecular Weight | 126.12 |
| InChI | InChI=1S/C4H6N4O/c5-3-2(4(6)9)7-1-8-3/h1H,5H2,(H2,6,9)(H,7,8) |
| InChI Key | DVNYTAVYBRSTGK-UHFFFAOYSA-N |
| Canonical SMILES | c1[nH]c(c(n1)C(=O)N)N |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US5728707 | Treatment and prevention of primary and metastatic neoplasms with salts of aminoimidazole carboxamide | 1998 |
| US6239137 | Salts of aminoimidazole carboxamide and 5-amino- or substituted amino-1,2,3-triazoles induce apoptosis, inhibit DNA synthesis and control cyclooxygenase activity. | 2001 |
| US5082829 | AICA riboside prodrugs | 1992 |
| US6184227 | Aminoimidazolecarboxamide salts useful in the prevention and treatment of liver diseases. | 2001 |
Physical Data
| Appearance | White to off-white crystalline powder |
| Solubility | Soluble in DMSO, Methanol, Water. |
| Refractive index | 1.8500 (estimate) |
| Melting Point, °C | Solvent (Melting Point) |
| 169 – 171 | |
| 172 – 174 | H2O |
| 170 | ethanol |
| 169.8 – 171.4 | ethanol, benzene |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | water-d2 | 300 | |
| Chemical shifts, Spectrum | 13C | water-d2 | 75 | |
| Chemical shifts | 1H | dimethylsulfoxide-d6, various solvent(s) | 37 | |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | ||
| Chemical shifts | 13C | dimethylsulfoxide-d6 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | KBr | 1680 cm**(-1) |
| Description (Mass Spectrometry) |
| electrospray ionisation (ESI), spectrum |
| high resolution mass spectrometry (HRMS), spectrum |
| fragmentation pattern, spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | 263 | |||
| Absorption maxima | H2O | Remark: pH 7, 0.005 M phosphate buffer | 268 | 11200 |
| Absorption maxima | H2O | Remark: pH 13, 0.1 N aq. NaOH | 278 | 12700 |
| Absorption maxima | ethanol, H2O | Ratio of solvents: 95:5 | 270 | 11500 |
| Absorption maxima | aq. HCl | Remark: pH 1 | 240, 267 | 7890, 9920 |
| Spectrum | acid / H2O | 220 – 300 nm | ||
| Spectrum | H2O | 220 – 300 nm, in neutraler wss.Loesung. | ||
| Absorption maxima | aq. HCl | 267 | ||
| Spectrum | aq. alkali | 220 – 300 nm |
Route of Synthesis (ROS)
| Conditions | Yield |
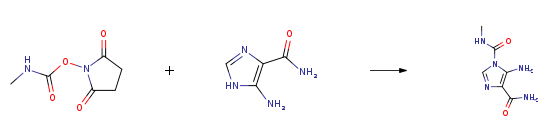
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 0 – 25℃; for 17h; Experimental Procedure 1 Synthesis of Carbamoyl AICA EXAMPLE 1 Synthesis of Carbamoyl AICA 200 g of base AICA as is (K. F. 11 %) corresponding to 178 g of 100% base AICA and 1000 ml of acetonitrile are charged in a 2-liter reactor. The mixture is stirred at room temperature (about 20° C.) and 267 g of N-Succinimidyl-N’-methyl carbamate and 191.7 g of diisopropylethylamine (DIPEA) are added to the suspension. The temperature of the mixture is kept at 25+2° C. for 16 hours, then the mixture is cooled at 0÷5° C., held for an hour and the suspension is filtered on a Buchner, by washing with 2*200 ml of deionised water. 313.0 g of wet product are discharged, which is then dried on a rotavapor for 5 hours at 50° C. with vacuum line. 212 g of Carbamoyl AICA (96.9% HPLC purity) are obtained, with 0.13% K. F. 88% yield. | 88% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (98.08%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (98.08%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (98.08%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | 293329 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Use Pattern |
| 5-Amino-4-imidazolecarboxamide CAS#: 360-97-4 inhibits dihydrofolate reductase(DHFR); thymidilate synthase (TMPS) inhibitor; GAR formyltransferase inhibitor; purine synthesis inhibitor; multi-target inhibitor |
| 5-Amino-4-imidazolecarboxamide CAS#: 360-97-4 Cardiac ischemia |
| 5-Amino-4-imidazolecarboxamide CAS#: 360-97-4 Potentiators of antifolate transport and metabolism |
| Cancers |
| Rheumatoid arthritis |
| Autoimmune diseases |
| Leukemia |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |