5-Bromo-7-azaindole CAS#: 183208-35-7; ChemWhat Code: 13855
Identification
| Product Name | 5-Bromo-7-azaindole |
| IUPAC Name | 5-bromo-1H-pyrrolo[2,3-b]pyridine |
| Molecular Structure | 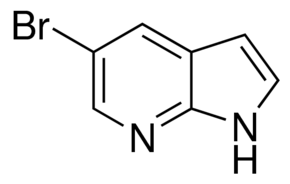 |
| CAS Registry Number | 183208-35-7 |
| EINECS Number | 629-247-8 |
| MDL Number | MFCD06659677 |
| Beilstein Registry Number | 8253948 |
| Synonyms | 5-bromo-1H-pyrrolo[2,3-b]pyridine, 5-Bromo-1H-pyrrolo[2,3-b]pyridine, tert-butyl 5-bromo-1H-pyrrolo[2,3-b]pyridine-1-carboxylate, 5-bromo-1H-pyrrolo<2,3-b>pyridine, 5-bromo-1H-pyrrolo[2, 3-b]pyridine, 5-bromo-1H-pyrrolo[2,3-b]-pyridine, 5-bromo-1H-pyrrolo(2,3-b)pyridine 5-Bromo-7-azaindole CAS#: 183208-35-7 CAS#: 183208-35-7 |
| Molecular Formula | C7H5BrN2 |
| Molecular Weight | 197.03 |
| InChI | InChI=1S/C7H5BrN2/c8-6-3-5-1-2-9-7(5)10-4-6/h1-4H,(H,9,10) |
| InChI Key | LPTVWZSQAIDCEB-UHFFFAOYSA-N |
| Canonical SMILES | C1=CNC2=NC=C(C=C21)Br |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2013/245355 | 3-HETARYL-SUBSTITUTED PYRROLO[2,3 B]PYRIDINE DERIVATIVES AS PDK1 INHIBITORS | 2013 |
| US2014/275082 | APOPTOSIS-INDUCING AGENTS FOR THE TREATMENT OF CANCER AND IMMUNE AND AUTOIMMUNE DISEASES | 2014 |
| US2015/11533 | 1H-PYRROLO[2,3-B] PYRIDINE DERIVATIVES AND THEIR USE AS KINASE INHIBITORS | 2015 |
| US2015/183781 | 5-(1H-Pyrazol-4-yl)-1H-Pyrrolo[2,3-b]Pyridine Derivatives as Kinase Inhibitors | 2015 |
| US2011/28511 | PROCESS FOR THE MANUFACTURE OF PHARMACEUTICALLY ACTIVE COMPOUNDS | 2011 |
Physical Data
| Appearance | White to yellow Crystalline Powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C | Solvent (Melting Point) |
| 176.8 – 177.3 | |
| 178.2 – 178.9 | |
| 174 – 176 | |
| 176 – 177 | pentane |
| Density, g·cm-3 | Measurement Temperature, °C | Type (Density) |
| 1.861 | 25.84 | crystallographic |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 300 |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 300 |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | |
| Chemical shifts | 1H | chloroform-d1 | 500 |
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Chemical shifts | 13C | chloroform-d1 | 100 |
| Spectrum | 1H | CDCl3 | 400 |
| Chemical shifts | 1H | CDCl3 | 400 |
| Chemical shifts | 13C | CDCl3 | 100 |
| Chemical shifts | 1H | CDCl3 | 300 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | potassium bromide | |
| ATR (attenuated total reflectance), Spectrum | ||
| Intensity of IR bands, Bands | ||
| ATR (attenuated total reflectance), Bands | ||
| Mid IR (MIR), Bands | potassium bromide | |
| Bands | KBr | |
| Bands | KBr | 3300 – 3000 1/cm |
| Description (Mass Spectrometry) | Comment (UV/VIS Spectroscopy) | Peak |
| liquid chromatography mass spectrometry (LCMS), spectrum | ||
| electron impact (EI), spectrum | ||
| high resolution mass spectrometry (HRMS), electron impact (EI), spectrum | ||
| LCMS (Liquid chromatography mass spectrometry) | Molecular peak | 198.04 m/z |
| ESI (Electrospray ionisation) | ||
| LCMS (Liquid chromatography mass spectrometry) | Molecular peak | |
| Molecular peak | ||
| Molecular peak | 197 m/z |
| Description (Raman Spectroscopy) |
| Bands, Spectrum |
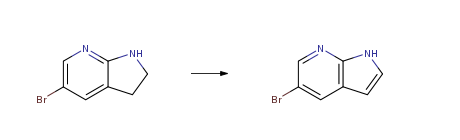
Route of Synthesis (ROS)
| Conditions | Yield |
| With manganese(IV) oxide In dichloromethane for 72h; Experimental Procedure To a stirred solution of azaindoline 5 (7.00 g, 35.2 mmol) in CH2C12 (664 ML) was added activated MnO2 (3.06 g, 35.2 MMOL), AND PROGRESS of the reaction was monitored BY LH EMR of reaction aliquots. After 3 days the mixture was filtered through A pad of silica, and the pad was washed with EtOAc. The filtrates were concentrated to afford the azaindole 27 (6. 98 g, 100 percent) as A brown solid. 1H NMR data as in Method 1. | 100% |
| With manganese(IV) oxide In toluene for 4h; Reflux; Experimental Procedure The 84.8 g of the 5-bromo-7-azaindoline product obtained in step 4 was dissolved in 400 mL of toluene,221.5 g of manganese dioxide was added,Heating reflux reaction 4h;Step 6) The reaction solution obtained in Step 5 was cooled to room temperature,filter,The filter cake was washed twice with dichloromethane,Combine organic phase,dry,Concentrated 5-bromo-7-azaindole crude product,The product was crystallized from a petroleum ether-ethyl acetate mixed solution of ΡΕ / ΕΑ = 10: 1 to give 75 g of 5-bromo-7-azaindole,Yield 90percent. | 90% |
| With activated carbon fiber catalyst In 5,5-dimethyl-1,3-cyclohexadiene at 100℃; for 8h; Solvent; Experimental Procedure a) 30g (0.151mol) of 5-bromo-7-azaporphyrin, 60g of activated carbon fiber catalyst, 264g of xylene into the reaction flask, stirring evenly, to obtain a reaction mixture G;b) The reaction mixture G at 100 °C, oxygen flow 200mL/min, reaction 8h, chromatographic monitoring of the disappearance of raw materials;c) Filtration, filter out activated carbon fiber catalyst, obtain organic layer H, recover solvent, get 5-bromo-7-azaindole crude product, recrystallize from methanol to obtain product 25.37g, yield 85.43percent, content ≥99percent . | 85.43% |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H302 (84.85%): Harmful if swallowed [Warning Acute toxicity, oral] H315 (18.18%): Causes skin irritation [Warning Skin corrosion/irritation] H318 (84.85%): Causes serious eye damage [Danger Serious eye damage/eye irritation] H319 (12.12%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (15.15%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P310, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | 293399 |
| Storage | Under the room temperature and away from light |
| Shelf Life | No data available |
| Market Price | USD |
| Use Pattern |
| 5-Bromo-7-azaindole CAS#: 183208-35-7 Used as Vemurafenib intermediate. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |