5-Pyrimidinylboronic acid CAS#: 109299-78-7; ChemWhat Code: 13749
Identification
| Product Name | 5-Pyrimidinylboronic acid |
| IUPAC Name | pyrimidin-5-ylboronic acid |
| Molecular Structure | 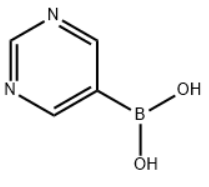 |
| CAS Registry Number | 109299-78-7 |
| EINECS Number | No data available |
| MDL Number | MFCD03002366 |
| Beilstein Registry Number | No data available |
| Synonyms | pyrimidine 5-boronic acidpyrimidin-5-yl boronic acid5-pyrimidineboronic acid5-pyrimidinylboronic acid5-pyrimidylboronic acidpyrimidine-5-ylboronic acidpyrimidinyl-5-boronic acid |
| Molecular Formula | C4H5BN2O2 |
| Molecular Weight | 123.908 |
| InChI | InChI=1S/C4H5BN2O2/c8-5(9)4-1-6-3-7-2-4/h1-3,8-9H |
| InChI Key | HZFPPBMKGYINDF-UHFFFAOYSA-N |
| Canonical SMILES | OB(O)c1cncnc1 |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2024/133014 | PHENYLPIPERIDINE DERIVATIVES AS INHIBITORS OF GLUTAMINYL-PEPTIDE CYCLOTRANSFERASE AND GLUTAMINYL-PEPTIDE CYCLOTRANSFERASE LIKE PROTEIN | 2024 |
| CN112375071 | Organic light-emitting compound and preparation method and application thereof | 2021 |
Physical Data
| Appearance | Off-white powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Spectrum | 11B | CD3OD | ||
| 1H | deuteromethanol | 250 | ||
| Chemical shifts | 1H | dimethylsulfoxide-d6 | ||
| Chemical shifts, Spectrum | 13C | dimethylsulfoxide-d6 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Spectrum | CCl4 | 14.85 – 54.85 |
| Description (Mass Spectrometry) |
| high resolution mass spectrometry (HRMS), electrospray ionisation (ESI), spectrum |
| high resolution mass spectrometry (HRMS), electrospray ionisation (ESI), spectrum |
| liquid chromatography mass spectrometry (LCMS), spectrum |
| LCMS (Liquid chromatography mass spectrometry) |
| CI (Chemical ionization) |
Route of Synthesis (ROS)
| Conditions | Yield |
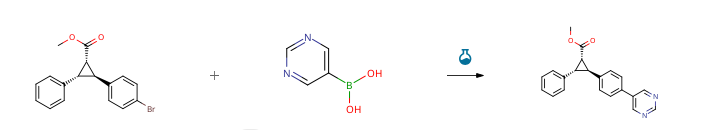
| With cesium fluoride; tetrakis(triphenylphosphine) palladium(0) In methanol; 1,2-dimethoxyethane at 120℃; for 1h; Inert atmosphere; microwave irradiation; | 93% |
| With tetrakis(triphenylphosphine) palladium(0); cesium fluoride In methanol; 1,2-dimethoxyethane at 120℃; for 1.25h; Inert atmosphere; Microwave irradiation; | 93% |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H318 (87%): Causes serious eye damage [Danger Serious eye damage/eye irritation] H319 (13%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (98.7%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P305+P354+P338, P317, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 123.907 |
| logP | -1.246 |
| HBA | 4 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 66.24 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Suzuki–Miyaura Coupling Reactions Widely used as a boronic acid building block in palladium-catalyzed cross-coupling reactions with aryl or vinyl halides. Enables the synthesis of pyrimidine-containing biaryl and heteroaryl compounds |
| Pharmaceutical Intermediates Serves as a key intermediate in the preparation of drug candidates, especially kinase inhibitors, antiviral agents, and anticancer compounds that incorporate the pyrimidine motif. |
| Agrochemical Synthesis Applied in the design and synthesis of heteroaryl derivatives used in herbicides, fungicides, and insecticides |
| Material Science Sometimes employed in the development of functional materials, such as conjugated heteroaryl polymers or ligands for coordination chemistry. |
| Medicinal Chemistry Research Useful for structure–activity relationship (SAR) studies, introducing pyrimidine groups into molecules to modulate biological activity and improve binding affinity. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |