5,6-DIHYDROXYINDOLE CAS#: 3131-52-0; ChemWhat Code: 361747
Identification
| Product Name | 5,6-DIHYDROXYINDOLE |
| IUPAC Name | 1H-indole-5,6-diol |
| Molecular Structure | 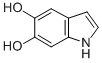 |
| CAS Registry Number | 3131-52-0 |
| EINECS Number | 412-130-9 |
| MDL Number | MFCD00798933 |
| Synonyms | 1H-indole-5,6-diol, 5,6-dihydroxy-indole, 5,6-dihydroxy indole, 5,6-Dihydroxyindole, 5,6-dihydroxyindol, CAP1194, CP1194 |
| Molecular Formula | C8H7NO2 |
| Molecular Weight | 149.149 |
| InChI | InChI=1S/C8H7NO2/c10-7-3-5-1-2-9-6(5)4-8(7)11/h1-4,9-11H |
| InChI Key | SGNZYJXNUURYCH-UHFFFAOYSA-N |
| Canonical SMILES | C1=CNC2=CC(=C(C=C21)O)O |
Physical Data
| Appearance | White crystalline powder |
| Melting Point, °C | Solvent (Melting Point) |
| 137 – 138 | benzene, petroleum ether |
| 143 – 144 | benzene, hexane |
| 138 – 139 | benzene, petroleum ether |
| 140 | benzene, light petroleum |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
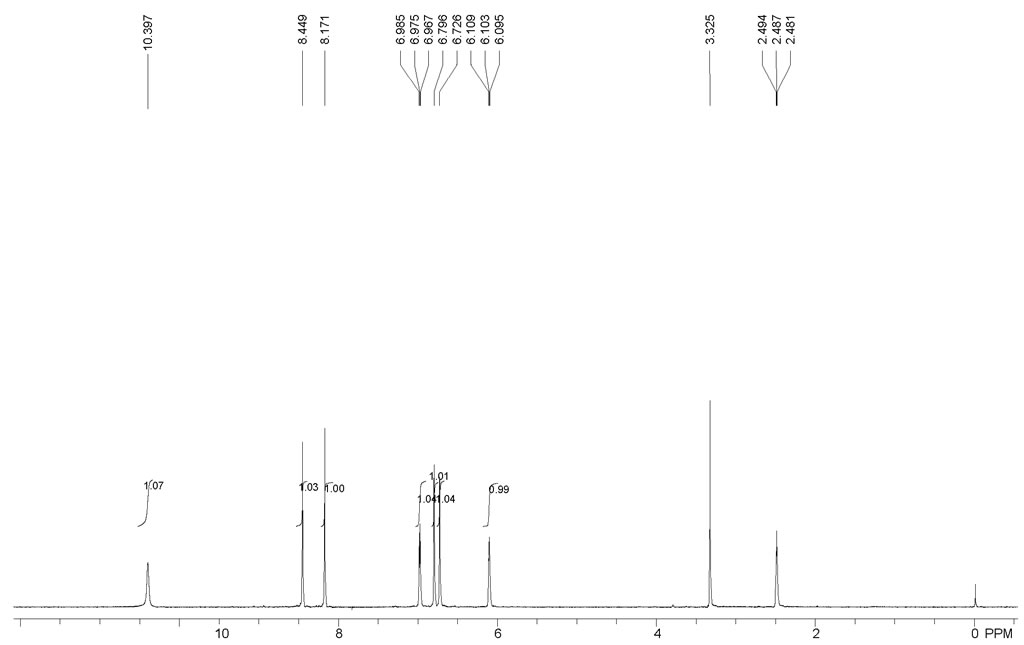
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | 400 |
| Chemical shifts | 13C | d(4)-methanol | 100 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands | KBr | 3436 – 486 cm**(-1) |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | methanol | 303 | 3340 |
| Absorption maxima | ethanol | 222, 276, 298 | 6310, 6310, 5623 |
Route of Synthesis (ROS)
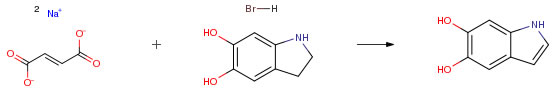
| Conditions | Yield |
| With sodium hydroxide; palladium-carbon In water Experimental Procedure 20 g of 5,6-dihydroxyindoline hydrobromide (86 mmoles) were dissolved in 500 ml of water and 14 g of fumaric acid disodium salt (87 mmoles) and 1.72 g of NaOH (43 mmoles) were successively added to the resulting solution. After addition of 4 g of 5percent Pd/C, the reaction mixture was refluxed for 1 hour in an inert gas atmosphere. After filtration, the reaction mixture was continuously extracted for 1 hour with t-butylmethyl ether. After drying of the organic phase over magnesium sulfate and subsequent filtration, the solvent was removed. 9.8 g (66 mmoles) of 5,6-dihydroxyindole were obtained in the form of a light beige-colored powder, corresponding to a yield of 76.5percent, based on the 5,6-dihydroxyindoline hydrobromide used. Analysis by HPLC showed that the 5,6-dihydroxyindole thus obtained has a purity of around 98percent. 1 -NMR (250 MHz, DMSO-d6): 6.12 ppm (d, J=3 Hz, 1H); 6.75 ppm (s, 1H); 6.82 ppm (s, 1H); 6.98 ppm (d, J=3 Hz, 1H); Determination of the coupling constants in MeOH-d4. | 76.5% |
Safety and Hazards
| Pictogram(s) |    |
| Signal | Danger |
| GHS Hazard Statements | H302: Harmful if swallowed [Warning Acute toxicity, oral] H318: Causes serious eye damage [Danger Serious eye damage/eye irritation] H411: Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P264, P270, P273, P280, P301+P312, P305+P351+P338, P310, P330, P391, and P501 (The corresponding statement to each P-code can be found at the?GHS Classification?page.) |
Other Data
| Transportation | Class 9; Packaging Group: III; UN Number: 3077 |
| Under 2- 8°C and away from light | |
| HS Code | 294200 |
| Storage | Under -15°C and away from light for long term storage |
| Shelf Life | 3-6 months(2- 8°C) and 1-2 years( -15°C) |
| Market Price | USD 8800/kg |
| Use Pattern |
| light-responsive self-tanning composition |
| self-tanning agent |
| dye precursor |
| air-oxidative hair dye |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Watson Noke Scientific Ltd | https://www.watsonnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |