6-Hydroxyindole CAS#: 2380-86-1; ChemWhat Code: 211766
Identification
| Product Name | 6-Hydroxyindole |
| IUPAC Name | 1H-indol-6-ol |
| Molecular Structure | 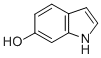 |
| CAS Registry Number | 2380-86-1 |
| EINECS Number | 417-020-4 |
| MDL Number | MFCD00152101 |
| Synonyms | 6-hydroxy-1H-indole, 6-monohydroxyindole, 6-hydroxyindole, 1H-indol-6-ol, 6-hydroxy-indole, indol-6-ol, 6-hydroxy indole |
| Molecular Formula | C8H7NO |
| Molecular Weight | 133.15 |
| InChI | InChI=1S/C8H7NO/c10-7-2-1-6-3-4-9-8(6)5-7/h1-5,9-10H |
| InChI Key | XAWPKHNOFIWWNZ-UHFFFAOYSA-N |
| Canonical SMILES | C1=CC(=CC2=C1C=CN2)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2014/275079 | PYRROLE AMIDE INHIBITORS | 2014 |
| WO2013/150529 | INDOLE, INDOLINE DERIVATIVES, COMPOSITIONS COMPRISING THEM AND USES THEREOF | 2013 |
| US2008/104774 | AMMONIA-FREE OXIDATION DYE FOR DYEING KERATIN FIBERS WITH ATMOSPHERIC OSYGEN SERVING AS THE SOLE OXIDIZING AGENT | 2008 |
| US5053053 | Process for dyeing keratinous fibres with a hydroxyindole in combination with a quinone derivative; and novel 1,4-benzoquinones | 1991 |
Physical Data
| Appearance | Off white solid |
| Melting Point, °C | Solvent (Melting Point) |
| 128 – 129 | CHCl3, ethyl acetate |
| 124 – 126 | CHCl3 |
| 125.5 | petroleum ether |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
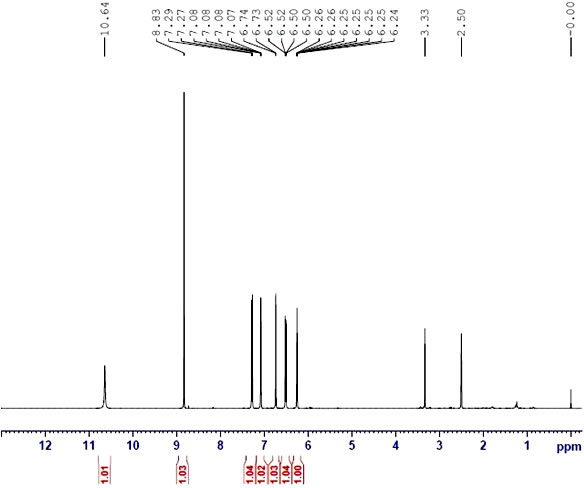
| Chemical shifts | 1H | deuteromethanol | 600 | |
| Chemical shifts | 13C | deuteromethanol | 151 | |
| Spectrum | 1H | dimethylsulfoxide-d6, aq. phosphate buffer, water-d2 | 26.84 | 600 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | KBr | 3400 – 720 cm**(-1) |
| Spectrum | nujol | 5000 – 667 cm**(-1) |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | Cyclohexane | 260, 267, 294, 301 | 5740 | |
| Absorption maxima | methanol | 218, 269, 294 | 35000, 4720, 6130 | |
| Spectrum | ethanol | 200 – 320 nm |
Route of Synthesis (ROS)
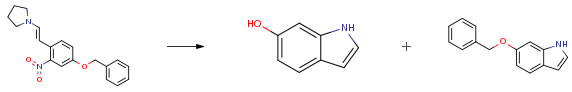
| Conditions | Yield |
| With hydrogen; 5% rhodium-on-charcoal; iron(II) acetate In tetrahydrofuran at 20℃; for 9h; Experimental Procedure Following a procedure similar to Example 32, 5-benzyloxy-2-(2-pyrrolidinylvinyl)nitrobenzene as a starting material was reduced in the presence of 5 percent rhodium/carbon powder and as a metal compound, ferrous(II) acetate, nickel(II) nitrate or cobalt(III) acetylacetonate as the catalyst of the present invention. The results are shown in the following Table 6, and in the case where the reducing agent of the present invention was used, 6-benzyloxyindole was obtained in a higher yield and 6-hydroxyindole was byproduced in a lower yield, compared to Comparison Example 5. | A 0.6% B 96% |
| With hydrogen; 5% rhodium-on-charcoal; tris(acetylacetonato)cobalt In tetrahydrofuran at 20℃; for 15h; Experimental Procedure Following a procedure similar to Example 32, 5-benzyloxy-2-(2-pyrrolidinylvinyl)nitrobenzene as a starting material was reduced in the presence of 5 percent rhodium/carbon powder and as a metal compound, ferrous(II) acetate, nickel(II) nitrate or cobalt(III) acetylacetonate as the catalyst of the present invention. The results are shown in the following Table 6, and in the case where the reducing agent of the present invention was used, 6-benzyloxyindole was obtained in a higher yield and 6-hydroxyindole was byproduced in a lower yield, compared to Comparison Example 5. | A 3% B 94% |
| With hydrogen; 5% rhodium-on-charcoal; nickel(II) nitrate In tetrahydrofuran; water at 20℃; for 23h; Experimental Procedure Following a procedure similar to Example 32, 5-benzyloxy-2-(2-pyrrolidinylvinyl)nitrobenzene as a starting material was reduced in the presence of 5 percent rhodium/carbon powder and as a metal compound, ferrous(II) acetate, nickel(II) nitrate or cobalt(III) acetylacetonate as the catalyst of the present invention. The results are shown in the following Table 6, and in the case where the reducing agent of the present invention was used, 6-benzyloxyindole was obtained in a higher yield and 6-hydroxyindole was byproduced in a lower yield, compared to Comparison Example 5. | A 1% B 90% |
Safety and Hazards
| Pictogram(s) |    |
| Signal | Danger |
| GHS Hazard Statements | H302: Harmful if swallowed [Warning Acute toxicity, oral] H317: May cause an allergic skin reaction [Warning Sensitization, Skin] H318: Causes serious eye damage [Danger Serious eye damage/eye irritation] H411: Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P270, P272, P273, P280, P301+P312, P302+P352, P305+P351+P338, P310, P321, P330, P333+P313, P363, P391, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class 9; Packaging Group: III; UN Number: 3077 |
| Under 2- 8°C and away from light | |
| HS Code | 294200 |
| Storage | Under -15°C and away from light for long term storage |
| Shelf Life | 3-6 months (2- 8°C) and 1-2 years( -15°C) |
| Market Price | USD 6800/kg |
| Use Pattern |
| coupler for dyeing keratin fibers |
| oxidative dye for hair colouring or bleaching composition |
| Dyeing of keratin fibers |
| coupler in composition for oxidation dyeing of keratin fibres |
| • Reactant for preparation of tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators • Reactant for asymmetrical synthesis of notoamide J as a potential biosynthetic precursor of prenylated indole alkaloids • Reactant for preparation of (quinolinyloxymethyl)isox azolecarboxylate esters antituberculosis agents • Reactant for preparation of indolyl(propanolamine) derivatives as HIV inhibitors • Reactant for preparation of indoleoxyacetic acid derivatives as peroxisome proliferator-activated receptor agonists • Reactant for preparation of 1-aroylindole 3-aroylindoles combretastatin A-4 analogs as antitumor agents and tubulin polymerization inhibitors |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Watson Noke Scientific Ltd | https://www.watsonnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |