| Official Full Name |
Recombinant Human Tumor Necrosis Factor-alpha/TNFSF2, Variant (rHuTNF-alpha/TNFSF2,Variant) |
| Squence |
 |
| Amino Acid Sequence |
MRKRKPVAHV VANPQAEGQL QWLNRRANAL LANGVELRDN QLVVPSEGLY LIYSQVLFKG QGCPSTHVLL THTISRIAVS YQTKVNLLSA IKSPCQRETP EGAEAKPWYE PIYLGGVFQL EKGDRLSAEI NRPDYLDFAE SGQVYFGIIA F |
| Synonyms |
Tumor Necrosis Factor, TNFSF2, Cachectin, Differentiation-inducing factor (DIF), Necrosin, Cytotoxin |
| Accession Number |
P01375 |
| GeneID |
7124 |
| Summary |
The clinical use of the potent antitumor activity of TNF-alphahas been limited by the proinflammatory side effects including fever, dose-limiting hypotension, hepatotoxicity, intravascular thrombosis, and hemorrhage. Designing clinically applicable TNF-alphamutants with low systemic toxicity has been an intense pharmacological interest. Human TNF-alpha, which binds to the murine TNF-R55 but not to the murine TNF-R75, exhibits retained antitumor activity and reduced systemic toxicity in mice compared with murine TNF-alpha, which binds to both murine TNF receptors. Based on these results, many TNF-alphamutants that selectively bind to TNF-R55 have been designed. These mutants displayed cytotoxic activities on tumor cell lines in vitro, and exhibited lower systemic toxicity in vivo. |
| Source |
Escherichia coli. |
| Molecular Weight |
Approximately 16.9 kDa, a single non-glycosylated polypeptide chain containing 151 amino acids. Compared with the wild-type, rHuTNF-α Variant has an amino acid sequence (a.a.) deletion from a.a. 1-7, and the following a.a. substitutes Arg8, Lys9, Arg10 and Phe157 which is proven to have more activity and with less inflammatory side effect in vivo. |
| Biological Activity |
Fully biologically active when compared to standard. The ED50 as determined by a cytotoxicity assay using murine L929 cells is less than 0.01 ng/ml, corresponding to a specific activity of > 1.0 × 107 IU/mg in the presence of actinomycin D. |
| Appearance |
Sterile filtered white lyophilized (freeze-dried) powder. |
| Formulation |
Lyophilized from a 0.2 um filtered concentrated solution in PBS, pH 7.0. |
| Endotoxin |
Less than 1 EU/ug of rHu TNF-α/TNFSF2, Variant as determined by LAL method. |
| Reconstitution |
We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Reconstitute in sterile distilled water or aqueous buffer containing 0.1 % BSA to a concentration of 0.1-1.0 mg/mL. Stock solutions should be apportioned into working aliquots and stored at ≤ -20 °C. Further dilutions should be made in appropriate buffered solutions. |
| Stability and Storage |
Use a manual defrost freezer and avoid repeated freeze-thaw cycles.- 12 months from date of receipt, -20 to -70 °C as supplied.- 1 month, 2 to 8 °C under sterile conditions after reconstitution.- 3 months, -20 to -70 °C under sterile conditions after reconstitution. |
| References |
|
| SDS-PAGE |
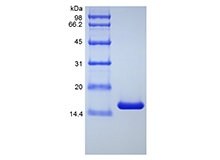 |
| Safety Data Sheet (SDS) Download |
Click to download |
| Technical Data Sheet (TDS) Download |
Click to download |



