Antimony tribromide CAS#: 7789-61-9; ChemWhat Code: 19084
Identification
| Product Name | Antimony tribromide |
| IUPAC Name | tribromostibane |
| Molecular Structure | 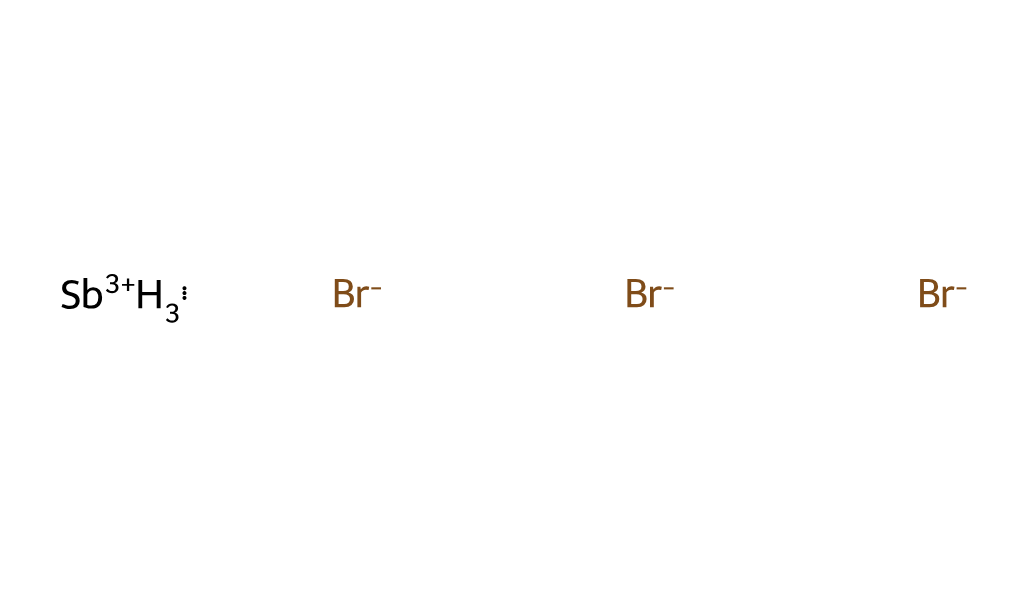 |
| CAS Registry Number | 7789-61-9 |
| EINECS Number | 232-179-8 |
| MDL Number | MFCD00006400 |
| Beilstein Registry Number | 105692 |
| Synonyms | antimony tribromideantimony(III) bromideantimony bromide |
| Molecular Formula | SbBr3 |
| Molecular Weight | 361.47 |
| InChI | InChI=1S/3BrH.Sb.3H/h3*1H;;;;/q;;;+3;;;/p-3 |
| InChI Key | BJUAQAQGPWXYGO-UHFFFAOYSA-K |
| Canonical SMILES | [Br-].[Br-].[Br-].[SbH3+3] |
| Patent Information |
| No data available |
Physical Data
| Appearance | Powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C | Solvent (Melting Point) |
| 122.84 | hexane |
| 55 – 57 | ethanol |
| 62 – 63 | aq. ethanol |
| 63 – 64 | benzene, petroleum ether |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 288 | 749 |
| 275 | 760 |
| 143 | 11 |
| 92 – 102 |
| Density, g·cm-3 | Measurement Temperature, °C | Comment (Density) |
| 3.6912 | 95 | Liquid; ref. temp. 4 Deg C |
| 1.3161 | 50 | 10 mol% SbBr3 in aniline; ref. temp. 4 Deg C |
| 1.7885 | 50 | 25 mol% SbBr3 in aniline; ref. temp. 4 Deg C |
Spectra
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C | Comment (IR Spectroscopy) |
| Bands | solid | 24.84 | 40 cm**-1 – 290 cm**-1 |
| Bands | solid matrix | -263.16 – -253.16 | 20 cm**-1 – 280 cm**-1 |
| Description (Mass Spectrometry) | Comment (Mass Spectrometry) |
| Spectrum | Molecular peak, Fragmentation pattern |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) |
| Spectrum, Band assignment | o-xylene=o-xylol | 250 nm – 384.615 nm |
| Spectrum, Band assignment | further solvent(s) | 250 nm – 384.615 nm |
| Spectrum, Band assignment | toluene | 250 nm – 384.615 nm |
Route of Synthesis (ROS)
| Conditions | Yield |
| With sulfuric acid In methanol at 100℃; for 3h; | 84.9% |
| In methanol |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Warning |
| GHS Hazard Statements | H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] H332 (100%): Harmful if inhaled [Warning Acute toxicity, inhalation] H411 (100%): Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P270, P271, P273, P301+P317, P304+P340, P317, P330, P391, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 361.462 |
| logP | 2.961 |
| HBA | 0 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 0 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Organic synthesis catalyst SbBr₃ can be used as a Lewis acid to catalyze Friedel-Crafts alkylation or acylation reactions in organic synthesis, especially for some synthetic routes that require mild conditions. Analytical reagent Used in analytical chemistry, especially as a colorimetric or precipitating agent when detecting certain organic compounds or metal ions. Preparation of other antimony compounds SbBr₃ can be used as an intermediate for the synthesis of other antimony compounds (such as antimonates, antimony organic complexes, etc.). Optoelectronic materials and semiconductor fields (special applications) Although less common, in the study of some special materials, SbBr₃ can be used to prepare antimony-containing bromide crystals or films for infrared detectors or photoconductive materials. Pigments or glass additives (limited to scientific research or special glass) May be added as a component to glass or ceramic materials to improve certain properties, such as refractive index, transparency, etc. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| WatsonChem Advanced Chemical Materials | https://www.watsonchem.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |