AZD-9291 CAS#: 1421373-65-0; ChemWhat Code: 82134
Identification
| Product Name | AZD-9291 |
| IUPAC Name | N-[2-[2-(dimethylamino)ethyl-methylamino]-4-methoxy-5-[[4-(1-methylindol-3-yl)pyrimidin-2-yl]amino]phenyl]prop-2-enamide |
| Molecular Structure | 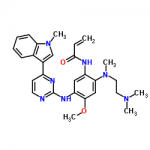 |
| CAS Registry Number | 1421373-65-0 |
| EINECS Number | No data available |
| MDL Number | No data available |
| Beilstein Registry Number | No data available |
| Synonyms | [N-(2-{[2-(dimethylamino)ethyl](methyl)amino}-4-methoxy-5-{[4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl]amino}phenyl)propen-2-amide], N-(2-{[2-(dimethylamino)ethyl](methyl)amino}-4-methoxy-5-{[4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl]amino}phenyl)prop-2-enamide, N-(5-(6-(1-methyl-1H-indol-3-yl)-pyrimidin-2-ylamino)-2-((2-(dimethylamino)-ethyl)-methylamino)-4-methoxyl-phenyl)-acrylamide, N-[2-[[2-(dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1H-indol-3-yl)-2-pyrimidinyl]amino]phenyl]-2-propenamide, N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-( 1-Methyl-1H-indol-3-yl)pyrimidin-2-yl)amino)phenyl)acrylamide, N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(1-methyl-1H-indol-3-yl)-pyrimidin-2-yl)amino)phenyl)acrylamide, N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl)amino)phenyl)acrylamide CAS NO.: 1421373-65-0 |
| Molecular Formula | C28H33N7O2 |
| Molecular Weight | 499.61 |
| InChI | InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) |
| InChI Key | DUYJMQONPNNFPI-UHFFFAOYSA-N |
| Canonical SMILES | Cn1cc(c2c1cccc2)c3ccnc(n3)Nc4cc(c(cc4OC)N(C)CCN(C)C)NC(=O)C=C |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2019/164947 | INHIBITORS OF EGFR AND METHODS OF USE THEREOF | 2019 |
| WO2019/164945 | PHARMACEUTICAL COMBINATIONS OF EGFR INHIBITORS AND METHODS OF USE THEREOF | 2019 |
| CN107793413 | Pyrimidine heterocyclic compound and its preparation method and application (by machine translation) | 2018 |
| CN107935995 | A novel 2 – anilino-pyrimidine derivatives and their use in the preparation of antineoplastic (by machine translation) | 2018 |
| CN108047205 | 2 – (2, 4, 5 – Substituted phenylamino) pyrimidine derivatives, their preparation method and its application in the preparation of antineoplastic (by machine translation) | 2018 |
Physical Data
| Appearance | White powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C | Solvent (Melting Point) | Crystallizes With | Amount (Melting Point), mol |
| 68.85 | |||
| 103.8 | diethyl ether | ||
| 113.6 | ethyl acetate | ||
| 50.1 | cyclohexane | ||
| 117.7 | water | 1 | |
| 135.3 | water, methanol | water | 1.25 |
| 135.7 | water | 0.25 | |
| 133.4 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz | Original Text (NMR Spectroscopy) |
| Chemical shifts | 1H | chloroform-d1 | 400 | |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 500 | |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 1H-NMR(DMSO-d6) δ:2.27(s, 6H), 2.31(t, 2H),2.71(s, 3H), 2.93(t, 2H),3.87(s, 3H), 3.88(s, 3H),5.76(dd, 1H), 6.26(dd, 1H),6.43(dd, 1H), 7.02(s, 1H),7.18-7.30(m, 3H), 7.54(d,1H), 8.15(s, 1H), 8.28(d,1H), 8.51(s, 1H), 8.67(s,1H), 8.71(s, 1H), 10.11(s,1H). | |
| Chemical shifts | 1H | water-d2 | 400 | |
| Chemical shifts | 13C | water-d2 | 100 | |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 500 | |
| Chemical shifts | 1H | d(4)-methanol | ||
| Chemical shifts | 13C | dimethylsulfoxide-d6 | 176 | |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 1H NMR: 2.21 (6H, s), 2.29 (2H, t), 2.72 (3H, s), 2.89 (2H, t), 3.86 (3H, s), 3.92 (3H, s), 5.77 (1H, dd), 6.27 (1H, dd), 6.43 (1H, dd), 7.04 (1H, s), 7.15 (1H, t), 7.20-7.27 (2H, m), 7.53 (1H, d), 7.91 (1H, s), 8.24 (1H, d), 8.33 (1H, d), 8.68 (1H, s), 9.14 (1H, s), 10.22 (1H, s); |
| Description (IR Spectroscopy) |
| ATR (attenuated total reflectance), Bands, Spectrum |
| Description (Mass Spectroscopy) |
| fragmentation pattern, spectrum |
| spectrum |
| electrospray ionisation (ESI), spectrum |
| electrospray ionisation (ESI), fragmentation pattern, spectrum |
| liquid chromatography mass spectrometry (LCMS), electrospray ionisation (ESI), spectrum |
| high resolution mass spectrometry (HRMS), electrospray ionisation (ESI), spectrum |
Route of Synthesis (ROS)
| Conditions | Yield |
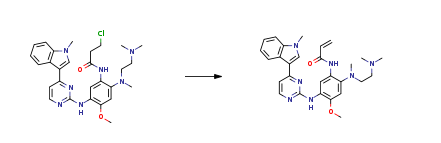
| With triethylamine In acetonitrile at 80℃; for 6h; Experimental Procedure 28.1 Example 28 (Alternative synthesis 1): N-(2-{2-Dimethylaminoethyl-methylamino}-4-methoxy-5-{[4-(1-methylindol-3-yl)pyrimidin-2-yl]amino}phenyl)prop-2-enamide To a stirred solution of 3-chloro-N-[2-[2-dimethylaminoethyl(methyl)amino]-4-methoxy-5-[[4-(1-methylindol-3-yl)pyrimidin-2-yl]amino]phenyl]propanamide (Intermediate 174, 31.5 g, 58.76 mmol) in acetonitrile (310 mL) was added triethylamine (17.84 g, 176.28 mmol) at r.t. The resulting mixture was heated to 80°C for 6h then cooled to r.t.. Water (130 mL) was then added and the mixture stirred for 12h. The mixture was then filtered, washed with a mixture of water and acetonitrile (160 mL, 1:1) and dried at 50°C for overnight to give the title compound (19.2 g, 94%) as a solid form identified herein as polymorphic form D.?1H NMR: 2.69 (3H, s), 2.83 (6H, d), 3.35 (4H, s), 3.84 (3H, s), 3.91 (3H, s), 5.75 (1H, d), 6.28 (1H, d), 6.67 (1H, dd), 7.05-7.23 (2H, m), 7.29 (1H, t), 7.43 (1H, d), 7.56 (1H, d), 8.21 (2H, s), 8.81 (1H, s), 9.47 (1H, s), 9.52 (1H, s), m/z: ES+?MH+?500.26. | 94% |
| With sodium hydroxide In tetrahydrofuran; water at 65℃; for 10h; Experimental Procedure (2-dimethylaminoethyl)-5-methoxy-N1-methyl-N4-[4-(1-methylindol-3-yl)pyrimidine at 0 ° C Yl) benzene-1,2,4-triamine (Intermediate 100, 10 g, 21.32 mmol) in THF (95 mL) and water (9.5 mL) was added 3-chloropropionyl chloride (3.28 G, 25.59 mmol). The mixture was stirred at room temperature for 15 minutes and then NaOH (3.48 g, 85.28 mmol) was added. The resulting mixture was heated to 65 ° C and maintained for 10 hours. The mixture was then cooled to room temperature and CH30H (40 mL) and water (70 mL) were added. The resulting mixture was stirred overnight. The resulting solid was collected by filtration, washed with water (25 mL) and dried at 50 ° C for 12 hours to give compound A (7.0 g, 94%) as a solid. | 94% |
| With triethylamine In acetonitrile at 20 – 80℃; for 6h; | 94% |
| With hydrogen In 2-methyltetrahydrofuran; water at 40℃; under 15001.5 Torr; for 24h; chemoselective reaction; | 94% |
| With triethylamine In acetonitrile at 80℃; Large scale; | 91.2% |
Safety and Hazards
| GHS Hazard Statements | Not dangerous goods |
| Precautionary Statement Codes | Not dangerous goods (The corresponding statement to each P-code can be found at the?GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 293399 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 499.616 |
| logP | 3.596 |
| HBA | 8 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 87.55 |
| Rotatable Bond (RotB) | 11 |
| Matching Veber Rules | 1 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (quant) | Unit | Target | Effect |
| 11.7 | IC50 | 0.002 | nM | Epidermal growth factor receptor (L858R):Wild/Epidermal growth factor receptor (T790M):Wild | |
| 9.7 | IC50 | 0.2 | nM | Epidermal growth factor receptor (L858R):Mutated | |
| 9 | IC50 | 0.001 | μM | Epidermal growth factor receptor:Mutated | inhibitory activity |
| 9 | inhibition rate | epidermal growth factor-activated receptor [human]:Mutated | antineoplastic agent | ||
| 8.92 | IC50 | 1.2 | nM | Epidermal growth factor receptor:Mutated | |
| 8.84 | Ki (inhibition constant) | 1.46 | nM | Epidermal growth factor receptor:Mutated | inhibitory activity |
| 8.39 | IC50(Tyr 1173 phosphorylation) | 4.1 | nM | Epidermal growth factor receptor [human]:Mutated | |
| 7.89 | IC50(cell proliferation) | 13 | nM | antineoplastic agent | |
| 7.82 | inhibition rate | polycomb repressive complex 2 (EZH2 mutant) [human]:Wild | antineoplastic agent |
| Quantitative Results | ||
| 1 of 10 | Effect | antineoplastic agent |
| Target | Polycomb Repressive Complex 2 (PRC2) [human]:Wild | |
| Substance action on target | Inhibitor | |
| Biological material | MDA-MB-453 cell line | |
| Measurement | at temperature 60 degree C | |
| 2 of 10 | Effect | antineoplastic agent |
| Biological material | NCI-H1975 cell line | |
| 3 of 10 | Effect | antineoplastic agent |
| Biological material | PC-9/GR cell line | |
| 4 of 10 | Biological material | NCI-H2122 cell line |
| Measurement | G0/G1 arrest | |
| 5 of 10 | Biological material | H1975 L858R/T790M |
| Measurement | G0/G1 arrest | |
| 6 of 10 | Biological material | PC-9 (EGFR-del19) |
| Measurement | G0/G1 arrest | |
| 7 of 10 | Biological material | PC-9 (EGFR-del19) |
| Measurement | G0/G1 arrest | |
| 8 of 10 | Biological material | NCI-H3255 (L858R) cell line |
| Measurement | G0/G1 arrest | |
| 9 of 10 | Biological material | A-549 cell line |
| Measurement | G0/G1 arrest | |
| 10 of 10 | Effect | antineoplastic agent |
| Biological material | NCI-H1975 cell line | |
| Assay Description | Growth inhibitory concentration of compound against mutant non-small cell lung cancer H1975 (L858R/T790M) cell line upon incubation at 37 degree C for 96 hrs determined by CellTiter-Glo assay | |
| Measurement | Cell viability |
| In vitro: Animal Model |
| Quantitative Results |
| pX | Parameter | Value (qual) | Dose | Effect |
| 1 | inhibition rate(Tumor regrowth) | Not active | 25 mg/kg | antineoplastic agent |
| 1 | survival rate increase | Not active | antineoplastic agent | |
| 1 | inhibition rate | Not active | 5mg/kg | antineoplastic agent |
| 1 | tumor relapse inhibition | Not active | 10 | antineoplastic agent |
| 1 | Percentage change | Not active | 10mg/kg | antineoplastic agent |
| 1 | survival time increase | Not active | 30mg/kg | antineoplastic agent |
| 1 | tumor volume change | Not active | 5mg/kg | antineoplastic agent |
| Use Pattern |
| AZD-9291 CAS#: 1421373-65-0 as EGFR tyrosine kinase inhibitor |
| AZD-9291 CAS#: 1421373-65-0 as Pharmaceuticals |
| treating cancer in combination with Axl inhibitor |
| treating head and neck cancer |
| EGFR inhibitor in combination with RET inhibitor |
| EGFR-mutant cancer |
| advanced EGFR T790M-positive non-small cell lung cancer |
| metastatic EGFR T790M-positive non-small cell lung cancer |
| a fibroblast growth factor receptor (FGFR) inhibitor |
| treating a subject suffering from non-small cell lung cancer (NSCLC) in combination with anti-cancer agent |
| Ph-like acute lymphoblastic leukemia |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |