BCN-OH CAS#: 1263166-90-0; ChemWhat Code: 1491512
Identification
| Product Name | BCN-OH |
| IUPAC Name | [(1R,8S)-9-bicyclo[6.1.0]non-4-ynyl]methanol |
| Molecular Structure | 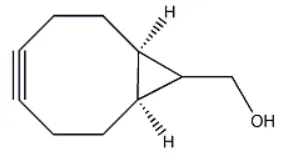 |
| CAS Registry Number | 1263166-90-0 |
| EINECS Number | No data available |
| MDL Number | MFCD26142970 |
| Beilstein Registry Number | 21037713 |
| Synonyms | (1R,8S,9s)-bicyclo[6.1.0]non-4-yn-9-ylmethanolBCNbicyclo[6.1.0]non-4-yn-9-ylmethanol(1R,8S,9r)-bicyclo[6.1.0]non-4-yn-9-ylmethanolendo-bicyclo[6.1.0]non-4-yn-9-ylmethanolendo-9-hydroxymethylbicyclo[6.1.0]non-4-yneendo-bicyclo[6.1.0]non-4-yn-9-ol |
| Molecular Formula | C10H14O |
| Molecular Weight | 150.221 |
| InChI | InChI=1S/C10H14O/c11-7-10-8-5-3-1-2-4-6-9(8)10/h8-11H,3-7H2/t8-,9+,10- |
| InChI Key | NSVXZMGWYBICRW-ILWJIGKKSA-N |
| Canonical SMILES | OC[C@H]1[C@H]2CCC#CCC[C@@H]12 |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2024/121123 | SYNTHESIS OF ENDO-BICYCLONONENE | 2024 |
| US2017/8858 | PROCESS FOR THE CYCLOADDITION OF A HALOGENATED 1,3-DIPOLE COMPOUND WITH A (HETERO)CYCLOALKYNE | 2017 |
Physical Data
| Appearance | White to off-white powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Chromatographic data |
| TLC (Thin layer chromatography) |
| HPLC (High performance liquid chromatography) |
| Comment (Solubility (MCS)) |
| has poor solubility in aqueous solutions |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
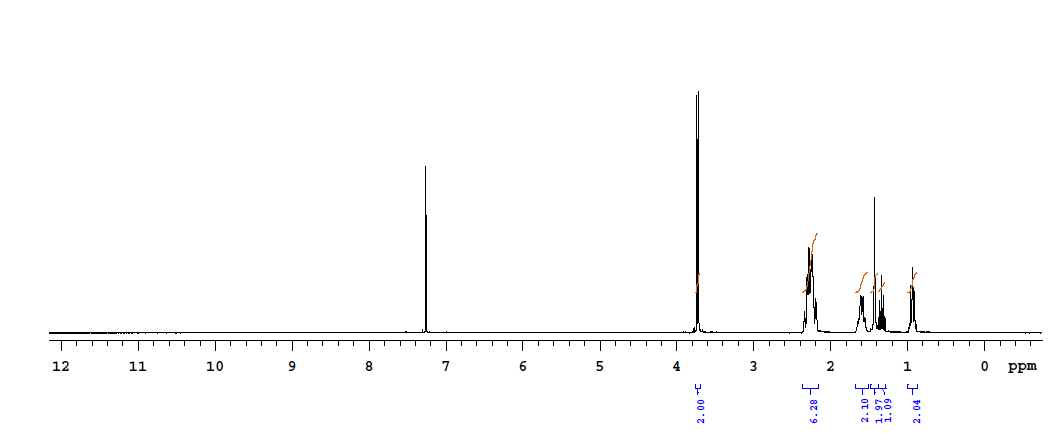
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 25 | |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 25 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 | |
| Spectrum | 1H | dimethylsulfoxide-d6, water-d2 | 400 |
| Description (Mass Spectrometry) |
| Spectrum |
| APCI (atmospheric pressure chemical ionization), spectrum |
| CI (Chemical ionization), high resolution mass spectrometry (HRMS), spectrum |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands, Spectrum | potassium bromide | film |
| Description (UV/VIS Spectroscopy) |
| Spectrum |
Route of Synthesis (ROS)
| Conditions | Yield |
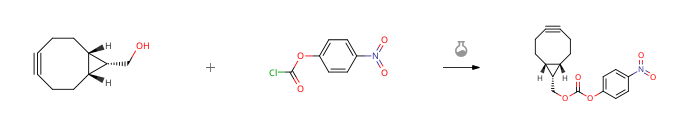
| With pyridine In dichloromethane | 94% |
| With pyridine In dichloromethane at 23℃; for 0.333333h; | 93% |
| With pyridine In N,N-dimethyl-formamide Inert atmosphere; | 89% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | WarningV |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501V (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Store at 2~8℃ for long time |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 150.221 |
| logP | 1.981 |
| HBA | 1 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 20.23 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Use Pattern |
| BCN-OH, a strained alkyne building block, is a mitochondrial probe based on the lyophilic bidentate bicyclic ligand BCN. The TPP group is a reactive sulfenic acid probe that targets mitochondria. It is primarily used in copper-free click chemistry and is commonly used for protein and antibody labeling and the preparation of bioprobes. |
| Biomolecule Modification and Labeling Protein/Antibody Labeling: BCN-OH derivatives are conjugated to azide-modified proteins or antibodies for imaging, diagnostics, or drug-drug conjugates (ADCs). Nucleic Acid and Carbohydrate Labeling: Conjugation to azide-modified oligonucleotides or polysaccharides for tracking or functionalization. |
| Drug Delivery and Nanomaterials Drug conjugates: They can serve as carriers for linking small molecule drugs to targeting vectors (e.g., antibodies or peptides) via click reactions. Nanoparticle Surface Modification: BCN-OH can be used to modify the surfaces of polymers, liposomes, or quantum dots, and then conjugated with azides for precise targeted delivery. |
| Materials Science Polymer Chemistry: Used as a monomer or cross-linker to construct functionalized polymers or hydrogels. Surface Modification: BCN groups are introduced onto glass, metal, and polymer surfaces, followed by click reactions with azides to achieve surface functionalization (e.g., biosensors and chips). |
| Diagnostics and Imaging Fluorescent Probes: Highly sensitive probes are obtained through click coupling with azide-containing fluorophores. Radioactive Probes: In molecular imaging, they are coupled with azide-containing radiolabels for PET/SPECT imaging. |
| Research Tools Metabolic Labeling: Azide-modified compounds (e.g., azidosugars) are introduced into cellular metabolism and then coupled with BCN to track cellular metabolic pathways. Protein Interaction Studies: Used as a cross-linking reagent to help immobilize or pull down target complexes. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |