BETA-NICOTINAMIDE MONONUCLEOTIDE CAS#: 1094-61-7; ChemWhat Code: 107733
Identification
| Product Name | BETA-NICOTINAMIDE MONONUCLEOTIDE |
| IUPAC Name | [(2R,3S,4R,5R)-5-(3-carbamoylpyridin-1-ium-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl hydrogen phosphate |
| Molecular Structure | 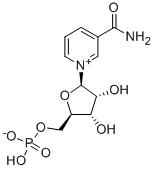 |
| CAS Registry Number | 1094-61-7 |
| EINECS Number | 214-136-5 |
| MDL Number | MFCD00038748 |
| Synonyms | Nicotinamide ribotide;[(2R,3S,4R,5R)-5-(3-Carbamoyl-1-pyridiniumyl)-3,4-dihydroxytetrahydro-2-furanyl]methyl hydrogen phosphate;1094-61-7 [RN];3-(Aminocarbonyl)-1-(5-O-phosphono-b-D-ribofuranosyl)pyridinium Hydroxide Inner Salt;3-Carbamoyl-1-[5-O-(hydroxyphosphinato)-β-D-ribofuranosyl]pyridinium [ACD/IUPAC Name];3-Carbamoyl-1-[5-O-(hydroxyphosphinato)-β-D-ribofuranosyl]pyridinium [German] [ACD/IUPAC Name];3-Carbamoyl-1-[5-O-(hydroxyphosphinato)-β-D-ribofuranosyl]pyridinium [French] [ACD/IUPAC Name];3-Carbamoyl-1-b-D-ribofuranosylpyridinium Hydroxide 5′-(Dihydrogen Phosphate) Inner Salt;Pyridinium,3-(aminocarbonyl)-1-(5-O-phosphono-β-D-ribofuranosyl)-, inner salt [ACD/Index Name];pyridinium, 3-(hydroxyiminomethyl)-1-(5-O-phosphono-β-D-ribofuranosyl)-, inner salt;3-(aminocarbonyl)-1-(5-O-phosphono-b-D-ribofuranosyl)-Pyridinium hydroxide inner salt;3-(aminocarbonyl)-1-(5-O-phosphono-b-D-ribofuranosyl)-Pyridinium inner salt;3-(aminocarbonyl)-1-(5-O-phosphono-β-δ-ribofuranosyl)-Pyridinium hydroxide inner salt;3-(aminocarbonyl)-1-(5-O-phosphono-β-δ-ribofuranosyl)-Pyridinium inner salt;3-Carbamoyl-1-b-D-ribofuranosylpyridinium hydroxide 5′-phosphate inner salt;3-Carbamoyl-1-β-δ-ribofuranosylpyridinium hydroxide 5′-phosphate inner salt;b-D-NMN;b-NMN;Nicotinamide ribonucleoside 5′-phosphate;β-δ-NMN;((2R,3S,4R,5R)-5-(3-Carbamoylpyridin-1-ium-1-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl hydrogen phosphate;??-Nicotinamide mononucleotide;?-Nicotinamide Mononucleotide;?-NMN;[(2R,3S,4R,5R)-5-(3-aminocarbonylpyridin-1-ium-1-yl)-3,4-dihydroxy-oxolan-2-yl]methyl hydrogen phosphate;[(2R,3S,4R,5R)-5-(3-carbamoyl-1-pyridin-1-iumyl)-3,4-dihydroxy-2-tetrahydrofuranyl]methyl hydrogen phosphate;[(2R,3S,4R,5R)-5-(3-carbamoylpyridin-1-ium-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl hydrogen phosphate;[(2R,3S,4R,5R)-5-(3-carbamoylpyridin-1-ium-1-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl]methyl hydrogen phosphate;¦Â-Nicotinamide mononucleotide;1-[(2R,3R,4S,5S)-3,4-dihydroxy-5-[(hydroxy-oxido-phosphoryl)oxymethyl]oxolan-2-yl]pyridine-5-carboxamide;1-[(2R,3R,4S,5S)-3,4-dihydroxy-5-[(hydroxy-oxido-phosphoryl)oxymethyl]tetrahydrofuran-2-yl]pyridine-5-carboxamide;3-(aminocarbonyl)-1-(5-O-phosphonato-β-D-ribofuranosyl)pyridinium;3-(aminocarbonyl)-1-(5-O-phosphono-?-D-ribofuranosyl)-pyridinium, inner salt;3-(aminocarbonyl)-1-(5-O-phosphono-β-D-ribofuranosyl)pyridinium, inner salt;3-(aminocarbonyl)-1-[5-O-(hydroxyphosphinato)-β-D-ribofuranosyl]pyridinium;3570187 [Beilstein];3-carbamoyl-1-[(2R,3R,4S,5R)-5-[(hydrogen phosphonatooxy)methyl]-3,4-dihydroxyoxolan-2-yl]-1λ5-pyridin-1-ylium;3-carbamoyl-1-[(2R,3R,4S,5R)-5-[(hydrogen phosphonatooxy)methyl]-3,4-dihydroxyoxolan-2-yl]-1λ5-pyridin-1-ylium;3-carbamoyl-1-β-D-ribofuranosylpyridinium hydroxide 5′-(dihydrogen phosphate) inner salt;B-NICOTINAMIDE MONONUCLEOTIDE;EINECS 214-136-5;MFCD00038748;Nicotinamide D-ribonucleotide;nicotinamide mononucleotide;Nicotinamide nucleotide;Nicotinamide ribonucleotide;Nicotinamide-1-ium-1-β-D-ribofuranoside 5′-phosphate;NMN;NMN zwitterion;UNII:2KG6QX4W0V;UNII-2KG6QX4W0V;β-Nicotinamide D-ribonucleotide;β-Nicotinamide mononucleotide;β-NICOTINAMIDE MONONUCLEOTIDE[4-3H(N)];β-Nicotinamide ribonucleotide;β-Nicotinamide ribose monophosphate;β-NICOTINAMIDEMONONUCLEOTIDE;β-NMN |
| Molecular Formula | C11H15N2O8P |
| Molecular Weight | 334.222 |
| InChI | InChI=1S/C11H15N2O8P/c12-10(16)6-2-1-3-13(4-6)11-9(15)8(14)7(21-11)5-20-22(17,18)19/h1-4,7-9,11,14-15H,5H2,(H3-,12,16,17,18,19)/t7-,8-,9-,11-/m1/s1 |
| InChI Key | DAYLJWODMCOQEW-TURQNECASA-N |
| Canonical SMILES | C1=CC(=C[N+](=C1)C2C(C(C(O2)COP(=O)(O)[O-])O)O)C(=O)N |
| Isomeric SMILES | C1=CC(=C[N+](=C1)[C@H]2[C@@H]([C@@H]([C@H](O2)COP(=O)(O)[O-])O)O)C(=O)N |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2018/334474 | NICOTINAMIDE MONONUCLEOTIDE DERIVATIVE AND SALT THEREOF, METHOD FOR PRODUCING SAME, TOPICAL SKIN PREPARATION, COSMETIC AND FOOD ADDITIVE | 2018 |
Physical Data
| Appearance | White to yellowish powder |
| Solubility | Methanol (Slightly), Water (Slightly) |
| Stability | Very Hygroscopic |
| Melting Point | >96°C (dec.) |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Stability constant of the complex with | D2O | 20 | bis(tetra-n-butylammonium) 6,8,15,17-tetrahydro-6,17:8,15-dimethanoheptacene-7,16-bismethylphosphonate |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
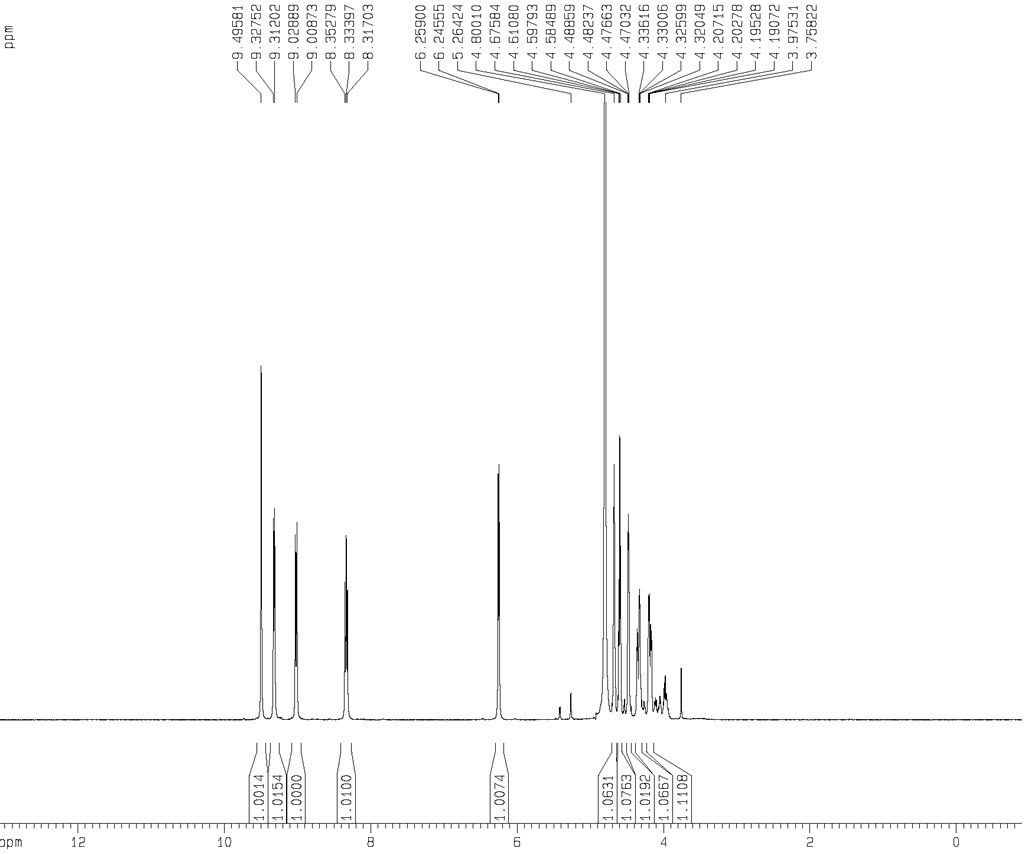
| Chemical shifts | 1H | water-d2 | 25 | 400 |
| Chemical shifts | 13C | water-d2 | 100 | |
| Chemical shifts | 31P | water-d2 | 25 | 162 |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Absorption maxima | 266 | 4200 | |
| Absorption maxima | H2O | 266 |
Route of Synthesis (ROS)
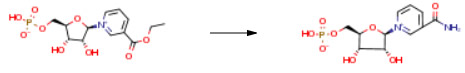
| Conditions | Yield |
| With ammonia In acetonitrile at -5 – 5℃; for 1h; Experimental Procedure -5 ° C, the content obtained in step c5′-nicotinic acid ethyl ester mononucleotide in an acetonitrile solution to react with ammonia gas,The reaction is exothermic, the reaction temperature is controlled below 5 ° C, and the ammonia gas is passed for about 30 minutes.Then, the reaction was stirred at -5 ° C for 30 min (Lc-Ms monitoring), and a large amount of sugar was precipitated.The acetonitrile layer was removed, then 20 mL of acetonitrile containing 2 mL of water was added and stirred at -5 ° C for 15 min.The acetonitrile layer was removed again, 20 mL of acetonitrile was added again, and stirred at -5 ° C for 15 min.The acetonitrile layer was removed to obtain an ammonium salt of a β-nicotinamide mononucleotide having a purity of 97percent of Lc-Ms.Removing the inorganic salt ammonium chloride and the like with a weakly basic anion exchange resin,Acidification removes excess ammonia,That is, an aqueous solution of β-nicotinamide mononucleotide having an HPLC purity of 97percent or more is obtained.Lyophilization gave 2.8 g of β-nicotinamide mononucleotide. The yield in this example was 80percent. | 80% |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Other Data
| Transportation | Not dangerous goods |
| Away from light and add ice packs for transportation. | |
| HS Code | 293499 |
| Storage | Keep in well-closed container under cold place (below 0°C) and away from direct sunlight, long term storage at -25℃ to -15℃ |
| Shelf Life | 1 year |
| Market Price | USD 5230/kg |
| Use Pattern |
| Pharmaceuticals |
| treatment of arteriosclerosis |
| treatment of diabetes |
| treatment of fatty liver disease |
| β-Nicotinamide mononucleotide (NMN) is used to study binding motifs within RNA aptamers and ribozyme activation processes involving β-nicotinamide mononucleotide (β-NMN)-activated RNA fragments. |
| treatment of hyperlipidemia |
| treatment of hypertension |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Ulcho Biochemical Ltd | https://www.ulcho.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |