Cannabidiol CAS#: 13956-29-1; ChemWhat Code: 50126
Identification
| Product Name | Cannabidiol |
| IUPAC Name | 2-[(1R,6R)-3-methyl-6-prop-1-en-2-ylcyclohex-2-en-1-yl]-5-pentylbenzene-1,3-diol |
| Molecular Structure | 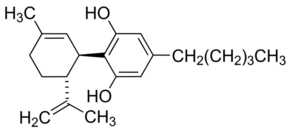 |
| CAS Registry Number | 13956-29-1 |
| EINECS Number | 200-659-6 |
| MDL Number | MFCD00869597 |
| Synonyms | (-)-CBD; (-)-Cannabidiol; (-)-trans-Cannabidiol; CBD;13956-29-1;CAS: 13956-29-1;CAS #:13956-29-1 |
| Molecular Formula | C21H30O2 |
| Molecular Weight | 314.46 |
| InChI | InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 |
| InChI Key | QHMBSVQNZZTUGM-ZWKOTPCHSA-N |
| Canonical SMILES | CCCCCC1=CC(=C(C(=C1)O)C2C=C(CCC2C(=C)C)C)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2020/16094 | THERAPEUTIC CANNABINOID DERIVATIVES COMPOSITION AS HISTAMINE 2 (H2) BLOCKING AGENTS | 2020 |
| US2020/69618 | COMPOSITIONS HAVING AN AGENT AND AN ENHANCER THEREOF, METHODS OF USE, AND DELIVERY SYSTEMS | 2020 |
| WO2020/41321 | PROCESS FOR THE PRODUCTION OF CANNABINOIDS | 2020 |
| US2019/23680 | SYNTHESIS OF CANNABINOIDS | 2019 |
| WO2018/94037 | ORAL THIN FILMS COMPRISING PLANT EXTRACTS AND METHODS OF MAKING AND USING SAME | 2018 |
Physical Data
| Appearance | Pale yellow resin or crystal |
| Solubility | No data available |
| Flash Point | 206.3℃ |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C | Solvent (Melting Point) | Crystallizes With |
| 65 – 67 | ||
| 43 – 47 | n-heptane | |
| 43 – 47 | n-heptane | |
| 64 – 65 | n-heptane | |
| 66 | hexane | |
| 66 – 67 | petroleum ether | |
| 66-67 | pentane |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 200 – 220 | |
| 179 – 183 | 0.08 |
| 187 – 190 | 2 |
| Density, g·cm-3 | Measurement Temperature, °C | Type (Density) |
| 1.109 | -173.16 | crystallographic |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Partner (Association (MCS)) |
| Stability constant of the complex with … | aq. phosphate buffer | hydroxypropyl-β-cyclodextrin (Cavasol(R) W7 HP) |
| Stability constant of the complex with … | aq. phosphate buffer | randomly methylated β-cyclodextrin (Cavasol(R) W7 M) |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | 400 |
| Chemical shifts, Spectrum | 13C | dimethylsulfoxide-d6 | 125 |
| Chemical shifts | 1H | deuteromethanol | 400 |
| Chemical shifts | |||
| Chemical shifts, Spectrum | 1H | deuteromethanol | 101 |
| NOE (Nuclear Overhauser Effect), Chemical shifts | 1H,1H | ||
| COSY (Correlation Spectroscopy), Spectrum | 1H,1H | ||
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 |
| Chemical shifts, Spectrum | 1H | water-d2 | 600 |
| Spectrum | 1H | d(4)-methanol | 400 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Original Text (IR Spectroscopy) |
| Bands, Spectrum | neat (no solvent, solid phase) | 27 |
| Bands | neat (no solvent, solid phase) | FT IR (cm−1): 3369 br, 2956, 2926, 2857, 1628, 1585, 1519, 1433, 1377, 1308, 1240, 1026, 960, 890, 864, 847, 829, 800, 658, 619 (partial reporting). |
| Spectrum | NaCl |
| Description (Mass Spectrometry) |
| high resolution mass spectrometry (HRMS), electrospray ionisation (ESI), time-of-flight mass spectra (TOFMS), spectrum |
| spectrum |
| electrospray ionisation (ESI), liquid chromatography mass spectrometry (LCMS), spectrum |
| liquid chromatography mass spectrometry (LCMS), spectrum |
| electrospray ionisation (ESI), time-of-flight mass spectra (TOFMS), tandem mass spectrometry, fragmentation pattern, spectrum |
| high resolution mass spectrometry (HRMS), spectrum |
| gas chromatography mass spectrometry (GCMS), spectrum |
| LCMS (Liquid chromatography mass spectrometry), Tandem mass spectrometry, ESI (Electrospray ionisation) |
| tandem mass spectrometry, electrospray ionisation (ESI), spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | acetonitrile, H2O | Ratio of solvents: 50/50 | ||
| Absorption maxima | ethanol | 274, 282 | 1300, 1300 | |
| UV/VIS | ||||
| Spectrum | ethanol | |||
| Spectrum | diethyl ether |
Route of Synthesis (ROS)
| Conditions | Yield |
| With hydrogen; platinum In ethyl acetate under 517.162 Torr; for 0.5h; | 97.5% |
| With platinum(IV) oxide; hydrogen In ethyl acetate at 20℃; under 517.162 Torr; for 0.0333333h; | 97.5% |
| With platinum(IV) oxide; hydrogen In ethyl acetate under 517.162 Torr; for 0.0333333h; | 97.5% |
Safety and Hazards
| Pictogram(s) |    |
| Signal | Danger |
| GHS Hazard Statements | H301 (11.11%): Toxic if swallowed [Danger Acute toxicity, oral] H302 (88.89%): Harmful if swallowed [Warning Acute toxicity, oral] H332 (11.11%): Harmful if inhaled [Warning Acute toxicity, inhalation] H361 (77.78%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] H413 (11.11%): May cause long lasting harmful effects to aquatic life [Hazardous to the aquatic environment, long-term hazard][Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P201, P202, P261, P264, P270, P271, P273, P281, P301+P310, P301+P312, P304+P312, P304+P340, P308+P313, P312, P321, P330, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class 3; Packaging Group: II; UN Number: 1230 |
| Under the room temperature and away from light | |
| HS Code | 293295 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 314.468 |
| logP | 6.804 |
| HBA | 0 |
| HBD | 2 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 40.46 |
| Rotatable Bond (RotB) | 6 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (quant) | Unit | Target |
| 11.3 | Ki (inhibition constant) | 0.0049 | nM | cannabinoid receptor 1 [mouse]:Wild |
| 11.2 | concentration (parameter) | 2 | pg/mL | |
| 9.78 | concentration (parameter) | 52 | pg/mL | |
| 9.56 | concentration (parameter) | 87 | pg/mL | |
| 9.5 | concentration (parameter) | 100 | pg/mL | |
| 9.4 | concentration (parameter) | 2.45 | pg/mL | |
| 9.35 | IC50 | 125 | nM | |
| 9.1 | concentration (parameter) | 249 | pg/mL | |
| 8.98 | concentration (parameter) | 326 | pg/mL |
| Quantitative Results | ||
| 1 of 10 | Effect | immunosuppressant antineoplastic agent |
| Biological material | Jiyoye cell line | |
| 2 of 10 | Effect | photoprotective agent |
| Biological material | human skin | |
| 3 of 10 | Effect | antagonistic activity |
| Target | G-protein coupled receptor 55 [human]:Wild | |
| Substance action on target | Antagonist | |
| Biological material | U2OS cell line | |
| Assay Description | Effective concentration of compound required for antagonistic activity towards human G PROTEIN-COUPLED RECEPTOR 55 in U2OS cells was measured by BETA-ARRESTIN TRANSLOCATION upon incubation for 40 mins | |
| Results | None | |
| Measurement | Beta-Arrestin Translocation | |
| 4 of 10 | Effect | binding activity |
| Target | cannabinoid receptor 2 [human]:Wild | |
| Substance action on target | Radioligand (/ligand) | |
| Biological material | HEK293 cell line | |
| Assay Description | Binding activity of compound towards human CANNABINOID RECEPTOR-2 in HEK 293 cells using [3H]CP-55940 as radioligand | |
| 5 of 10 | Effect | binding activity |
| Target | cannabinoid receptor 1 [rat]:Wild | |
| Biological material | brain membranes | |
| Substance action on target | Radioligand (/ligand) | |
| Assay Description | Binding activity of compound towards rat CANNABINOID RECEPTOR-1 in rat brain membranes using [3H]CP-55940 as radioligand | |
| 6 of 10 | Effect | inhibitory activity |
| Target | cannabinoid receptor 2 [human]:Wild | |
| Substance action on target | Inhibitor | |
| Biological material | SK-N-MC cell line | |
| Assay Description | Inhibitory activity against human Cannabinoid receptor 2 expressed in SK-N-MC cells was determined upon incubation for 90 min at 30 degree C with 0.2 nM of [3H]-CP-55940 as radioligand | |
| Results | Not Published | |
| 7 of 10 | Effect | inhibitory activity |
| Target | cannabinoid receptor 1 [human]:Wild | |
| Substance action on target | Inhibitor | |
| Biological material | SK-N-MC cell line | |
| Assay Description | Inhibitory activity against human Cannabinoid receptor 1 expressed in SK-N-MC cells was determined upon incubation for 90 min at 30 degree C with 0.2 nM of [3H]-CP-55940 as radioligand | |
| Results | Not Published | |
| 8 of 10 | Effect | binding activity |
| Target | Cannabinoid receptor type 1:Wild | |
| Assay Description | Binding affinity of compound towards cannabinoid receptor 1 was determined | |
| 9 of 10 | Effect | antibiotic agent |
| Target | Cannabinoid receptor type 2:Wild | |
| Assay Description | Binding affinity of compound towards cannabinoid receptor 2 was determined | |
| 10 of 10 | Target | estrogen receptor:Wild |
| Assay Description | Modulatory activity of the compound towards estrogen receptor was determined |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Effect |
| 8 | Decrease rate | Active | antineoplastic agent | ||
| 7.52 | IC50 | 0.03 | μM | antineoplastic agent | |
| 7.2 | LOAEC | 20 | μg/l | Toxic | |
| 7.02 | mRNA expression level decrease | Active | Toxic | ||
| 6.95 | IC50 | 0.26 | μM | antineoplastic agent | |
| 6.28 | inhibition rate(proliferation) | 97.92 | % | Neurotoxic | |
| 6.02 | LOAEC | 300 | μg/l | Toxic | |
| 5.8 | inhibition rate(proliferation) | 97.47 | % | antineoplastic agent |
| Use Pattern |
| In the United States, the cannabidiol drug Epidiolex was approved by the Food and Drug Administration in 2018 for the treatment of two epilepsy disorders. Since cannabis is a Schedule I controlled substance in the United States, other CBD formulations remain illegal to prescribe for medical use or to use as an ingredient in foods or dietary supplements. |
| Cannabidiol CAS#: 13956-29-1 in combination with a chelating agent, most preferably EDTA |
| Cannabidiol CAS#: 13956-29-1 infection is a skin infection |
| pain in combination wth tetrahydrocannabinol and terpene fraction from extract of Cannabis |
| part of composition for treating PTSD and/or anxiety |
| Cannabidiol CAS#: 13956-29-1 diffuse idiopathic skeletal hyperostosis (DISH) |
| Cannabidiol CAS#: 13956-29-1 treating an inflammatory disease in combination with 4-methylthiobutyl isothiocyanate (MTBI) and Angelica Sinensis extract |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |