CHLOROTITANIUM TRIISOPROPOXIDE CAS#: 20717-86-6; ChemWhat Code: 35740
Identification
| Product Name | CHLOROTITANIUM TRIISOPROPOXIDE |
| IUPAC Name | propan-2-olate;titanium(4+);chloride |
| Molecular Structure | 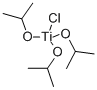 |
| CAS Registry Number | 20717-86-6 |
| MDL Number | MFCD00009861 |
| Synonyms | 20717-86-6 Titanium, chlorotris(2-propanolato)-, (T-4)- Chlorotriisopropoxytitanium W1IT56N129 UNII-W1IT56N129 CLTI(OI-PR)3 triisopropoxytitanium chloride TRIISOPROPOXYCHLOROTITANIUM TRIISOPROPOXYCYCLOCHLOROTITANIUM CHLOROTITANIUM TRIS(ISOPROPOXIDE) (T-4)-Chlorotris(2-propanolato)titanium CHLOROTITANIUM TRIISOPROPOXIDE [MI] TITANIUM MONOCHLORIDE TRIISOPROPOXIDE Chlorotrisopropylorthotitanate chlorotitanium triisopropoxide ifmwvbvpvxrzhe-uhfffaoysa-m propan-2-olate;titanium(4+);chloride SCHEMBL438557 DTXSID10942927 AKOS025394509 Titanium(4+) chloride propan-2-olate (1/1/3) |
| Molecular Formula | C9H21ClO3Ti |
| Molecular Weight | 260.579 |
| InChI | InChI=1S/3C3H7O.ClH.Ti/c3*1-3(2)4;;/h3*3H,1-2H3;1H;/q3*-1;;+4/p-1 |
| InChI Key | IFMWVBVPVXRZHE-UHFFFAOYSA-M |
| SMILES | CC(C)[O-].CC(C)[O-].CC(C)[O-].[Cl-].[Ti+4] |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| JP2016/147819 | GROUP 4 METAL COMPLEX, PRODUCTION METHOD THEREOF, PREPARATION METHOD OF GROUP 4 METAL-CONTAINING THIN FILM | 2016 |
| US2013/143843 | ARYL DIHYDROPYRIDINONE AND PIPERIDINONE MGAT2 INHIBITORS | 2013 |
| EP2412694 | PROCESS FOR PRODUCING OPTICALLY ACTIVE ALCOHOL | 2012 |
| US5347039 | Process for the preparation of derivatives of 3,5-dihydroxypentanoic acid | 1994 |
Physical Data
| Appearance | Colorless to yellow liquid or low melting solid |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) |
| Spectrum | 1H | not given |
Route of Synthesis (ROS)
| Conditions | Yield |
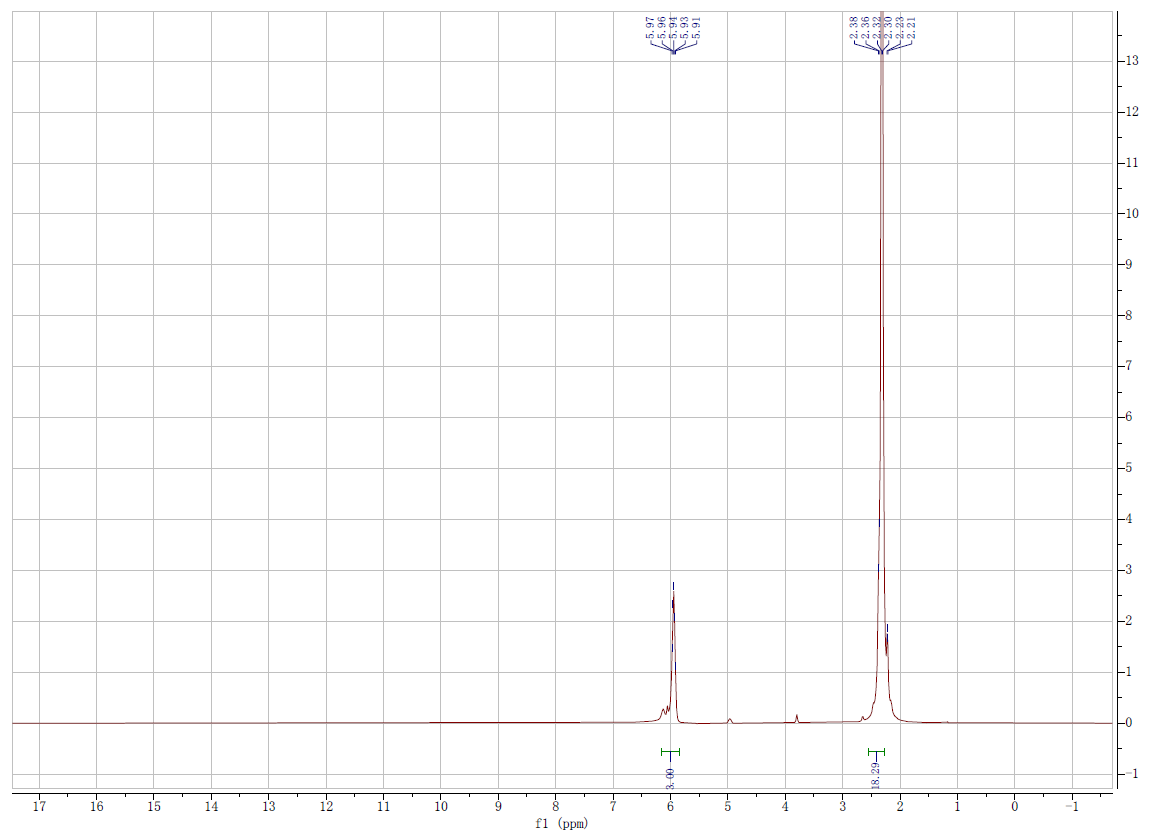
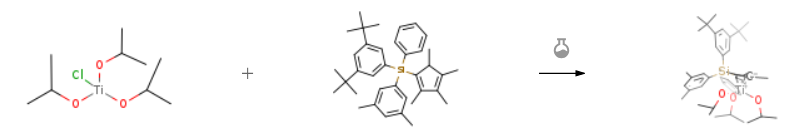
| Stage #1: 1-(3,5-di-tert-butylphenyl)(3,5-dimethylphenyl)(phenyl)silyl-2,3,4,5-tetramethylcyclopentadiene With potassium hydride In tetrahydrofuran at 25 – 50℃; for 7h; Inert atmosphere; Stage #2: triisopropoxytitanium(IV) chloride In tetrahydrofuran at -78 – 25℃; Experimental Procedure Synthesis of complex 2″Under a nitrogen atmosphere, potassium hydride dispersed in mineral oil was washed with hexane to remove the mineral oil. Thereafter, the resultant potassium hydride (0.26 g, 6.40 mmol) and tetrahydrofuran (20 mL) were mixed. To this mixture, a solution of 1- (3,5-di-tert-butylphenyl)(3,5-dimethylphenyl)(phenyl)silyl-2,3,4,5-tetramethylcyclopentadiene (2.57 g, 4.92 mmol) dissolved in tetrahydrofuran (28 mL) was added dropwise at room temperature and then stirred at 50°C for 7 hours. The resultant mixture was cooled to room temperature and insoluble materials were removed by filtration. The resultant filtrate was cooled to -78°C and a solution of chlorotriisopropoxytitanium (1.28 g, 4.92 mmol) dissolved in tetrahydrofuran (26 mL) was added dropwise at the same temperature. After the mixture was gradually warmed to room temperature, stirring was performed at room temperature overnight. After completion of the reaction, the solvent was removed under reduced pressure. Thereafter, pentane was added to the residue, and insoluble materials were removed by filtration. The solvent was removed from the filtrate under reduced pressure to obtain [l-(3,5-di-tert- butylphenyl)(3,5-dimethylphenyl)(phenyl)silyl-2,3,4,5-tetramethylcyclopentadienyl]titanium triisopropoxide (3.41 g, yield 92.9%).1H-NMR(CDC13, δ ppm): l.ll(dd, J=5.9, 1.5Hz, 18H), 1.23(s, 18H), 1.67(s, 6H), 1.99(s, 6H), 2.45(s, 6H), 4.55(sep, J=5.9Hz, 3H), 6.96(s, 1H), 7.20-7.44(m, 8H), 7.46(dd, J=7.7, 1.8Hz, 2H) 13C-NMR(CDC13, δ ppm): 11.59, 11.64, 14.87, 21.44, 26.46, 31.39, 34.77, 75.58, 112.70, 122.33, 126.35, 126.65, 127.13, 128.56, 130.52, 131.42, 131.60, 134.32, 134.42, 136.19, 136.72, 136.81, 137.24, 148.83Mass spec (EI-MS, m/z): 744(M+) | 92.9% |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H228 (100%): Flammable solid [Danger Flammable solids] H314 (97.8%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] |
| Precautionary Statement Codes | P210, P240, P241, P260, P264, P280, P301+P330+P331, P302+P361+P354, P304+P340, P305+P354+P338, P316, P321, P363, P370+P378, P405, and P501 |
Other Data
| Transportation | Under the room temperature and away from light |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 260.597 |
| logP | 3.317 |
| HBA | 3 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 27.69 |
| Rotatable Bond (RotB) | 6 |
| Matching Veber Rules | 2 |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Limited | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |