D-ERYTHRO-SPHINGOSINE-1-PHOSPHATE CAS#: 26993-30-6; ChemWhat Code: 277679
Identification
| Product Name | D-ERYTHRO-SPHINGOSINE-1-PHOSPHATE |
| IUPAC Name | [(E,2S,3R)-2-amino-3-hydroxyoctadec-4-enyl] dihydrogen phosphate |
| Molecular Structure | |
| CAS Registry Number | 26993-30-6 |
| EINECS Number | No data available |
| MDL Number | No data available |
| Beilstein Registry Number | No data available |
| Synonyms | Sphingosine-1-phosphate |
| Molecular Formula | C18H38NO5P |
| Molecular Weight | 379.478 |
| InChI | InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14+ |
| InChI Key | DUYSYHSSBDVJSM-CCEZHUSRSA-N |
| Canonical SMILES | CCCCCCCCCCCCC/C=C/C(O)C(N)COP(=O)(O)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2023/192956 | SPHINGOLIPID-LOADED NANOBIOLOGICS FOR IMMUNE REGULATION | 2023 |
Physical Data
| Appearance | White to light yellow powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz | Original Text (NMR Spectroscopy) |
| Chemical shifts | 1H | CD3OD, tetradeuterioacetic acid | 400 | ’H NMR (400 MHz, Methanol-d4+ CD3CO2D) δ 5.84 (dt, 7 = 15.5, 6.7 Hz, 1H), 5.46 (dd, J = 15.5, 6.7 Hz, 1H), 4.33 (t, 7 = 6.0 Hz, 1H), 4.13 (ddd, 7 = 11.8, 7.7, 3.6 Hz, 1H), 4.03 (dt, 7 = 11.8, 8.4 Hz, 1H), 3.47 (ddd, 7 = 8.3, 4.8, 3.2 Hz, 1H), 2.10 – 1.99 (m, 2H), 1.37 (m, 2H), 1.24 (m, 20H), 0.83 (t, 7 = 6.4 Hz, 3H) |
| Chemical shifts | 31P | chloroform-d1 | 162 | 31P NMR (162 MHz, Chloroform-d) 5 0.69 |
| Chemical shifts | 1H | CD3OD | 600 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | neat (no solvent) |
| Bands | KBr |
| Description (Mass Spectrometry) |
| high resolution mass spectrometry (HRMS), liquid chromatography mass spectrometry (LCMS), spectrum |
| high resolution mass spectrometry (HRMS), spectrum |
| liquid chromatography mass spectrometry (LCMS), electrospray ionisation (ESI), spectrum |
| time-of-flight mass spectra (TOFMS), liquid chromatography mass spectrometry (LCMS), electrospray ionisation (ESI), spectrum |
Route of Synthesis (ROS)
| Conditions | Yield |
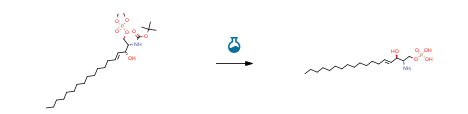
| With trimethylsilyl bromide In dichloromethane at 0 – 20℃; for 6h; | 83% |
| With trimethylsilyl bromide In dichloromethane at 0 – 20℃; for 6h; | 83% |
| With trimethylsilyl bromide In dichloromethane at 0 – 20℃; for 6h; | 83% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | 290621 |
| Storage | Storage at -20° |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 379.477 |
| logP | 4.789 |
| HBA | 6 |
| HBD | 4 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 122.82 |
| Rotatable Bond (RotB) | 17 |
| Matching Veber Rules | 1 |
| Use Pattern |
| Signal Molecule Research -Acts as a potent extracellular signaling lipid. -Binds to S1P receptors (S1PR1–5) and regulates cell migration, proliferation, differentiation, vascular development, and immune cell trafficking. |
| Pharmacological Research -Involved in immune regulation, cancer progression, cardiovascular diseases, and inflammation. -Basis for therapeutic development (e.g., Fingolimod/Gilenya®, an S1P receptor modulator for multiple sclerosis). |
| Research Reagent – Commonly used in cell signaling studies, lipid metabolism research, and immunology experiments. – Applied to study S1P-S1PR pathways in inflammation, tumor metastasis, and angiogenesis. |
| Potential Applications -Investigated as a drug target or tool compound for autoimmune diseases, atherosclerosis, asthma, and cancer therapy. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Ulcho Biochemical Ltd | http://www.ulcho.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |