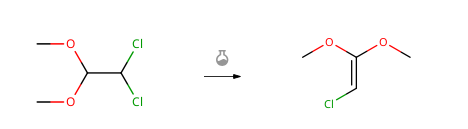
DCADMA CAS#: 80944-06-5; ChemWhat Code: 1491412
Identification
| Product Name | DCADMA |
| IUPAC Name | 1,1-dichloro-2,2-dimethoxyethane |
| Molecular Structure | 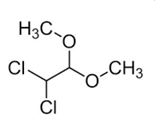 |
| CAS Registry Number | 80944-06-5 |
| EINECS Number | No data available |
| MDL Number | No data available |
| Beilstein Registry Number | No data available |
| Synonyms | Dichloracetaldehyd-dimethylacetaldichloro-acetaldehyde dimethylacetalβ.β-Dichlor-α.α-dimethoxy-aethan |
| Molecular Formula | C4H8Cl2O2 |
| Molecular Weight | 159.01 |
| InChI | InChI=1S/C4H8Cl2O2/c1-7-4(8-2)3(5)6/h3-4H,1-2H3 |
| InChI Key | NGVTXINFTCZHGA-UHFFFAOYSA-N |
| Canonical SMILES | COC(C(Cl)Cl)OC |
| Patent Information |
| No data available |
Physical Data
| Appearance | Clear Colorless to yellow Liquid |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 56 – 58 | 15.001 |
| 161 – 163 | 740 |
| 166 – 168 |
| Refractive Index | Wavelength (Refractive Index), nm | Temperature (Refractive Index), °C |
| 1.4405 | 589 | 25 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Comment (NMR Spectroscopy) |
| Chemical shifts, Spectrum | 1H | CDCl3 | |
| Spin-spin coupling constants | CDCl3 | 1H-1H |
| DCADMA CAS#: 80944-06-5 NMR | 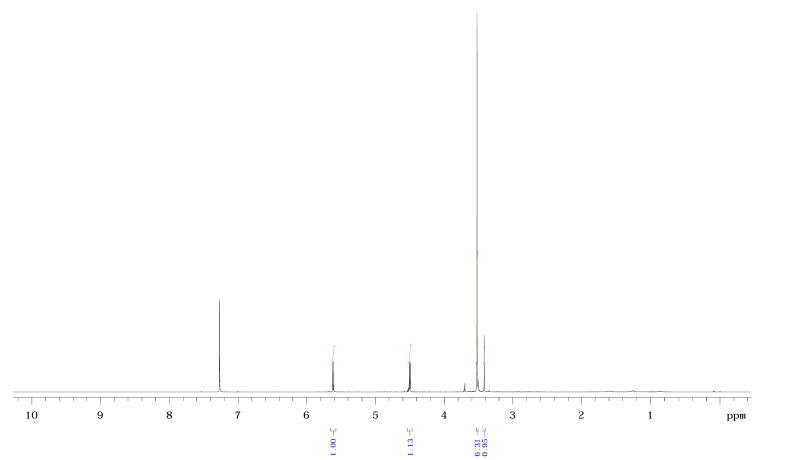 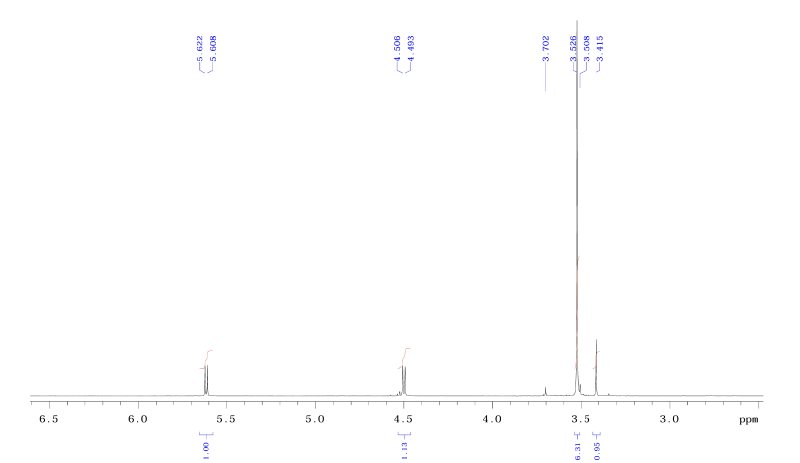 |
| DCADMA CAS#: 80944-06-5 IR | 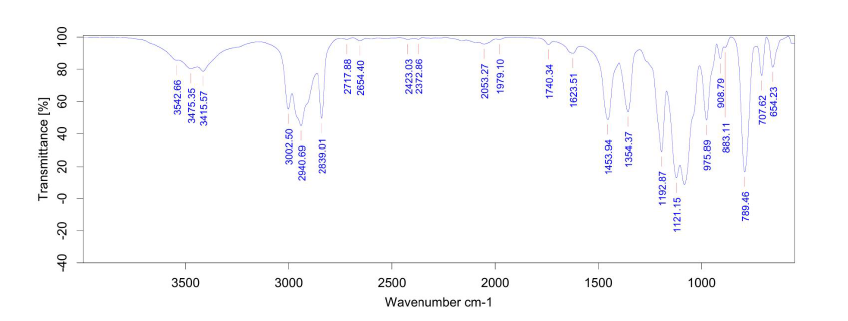 |
| DCADMA CAS#: 80944-06-5 MS | 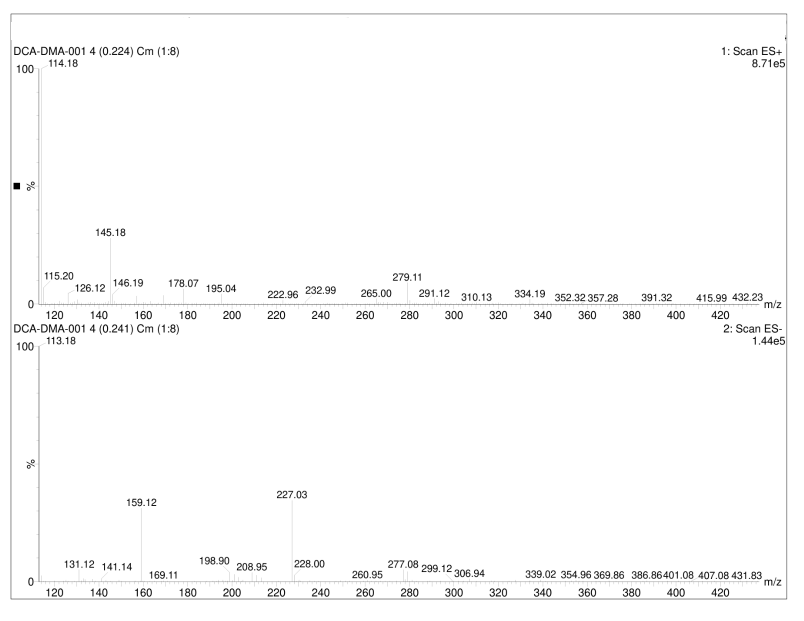 |
| DCADMA CAS#: 80944-06-5 GC | 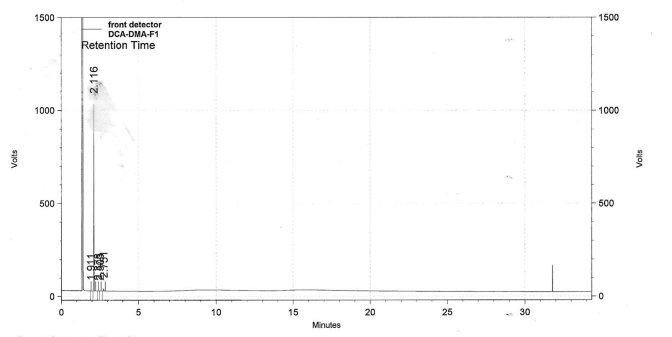 |
Route of Synthesis (ROS)
| Conditions | Yield |
| With potassium tert-butylate |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Other Data
| Transportation | NONH for all modes of transport |
| Store at 2 to 8 °C temperature, tightly closed container | |
| HS Code | No data available |
| Storage | Store at 2 to 8 °C temperature, tightly closed container |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 159.012 |
| logP | 1.187 |
| HBA | 2 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 18.46 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Used as the Praziquantel impurity 6, Trichlorfon lmpurity 1 or Herbicide intermediate. |
| Antibacterial/antistatic coatings and surface modifiers The quaternary ammonium salt structure gives it excellent antibacterial properties and can kill bacteria, fungi, and viruses; it can be introduced into coatings, films, gels, and fiber surfaces through copolymerization to form a long-lasting antibacterial layer; it is commonly used in medical devices, textile surface treatment, dental materials, contact lens surface modification, etc. |
| Polymer modified monomers The dimethacrylate group allows it to copolymerize with free radicals such as acrylic acid and methacrylate resins; it is used to prepare functional polymer materials such as hydrogels, water treatment membranes, and conductive coatings; it has special applications in 3D printing, biomedical polymers, and hydrogel systems. |
| Water treatment or membrane material additives The quaternary ammonium salt structure has ion exchange capacity and adsorption capacity for negatively charged pollutants; it can be used for anti-pollution modification of reverse osmosis membranes and flocculant structure construction; it improves the anti-pollution and hydrophilicity of the membrane and increases its service life. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |