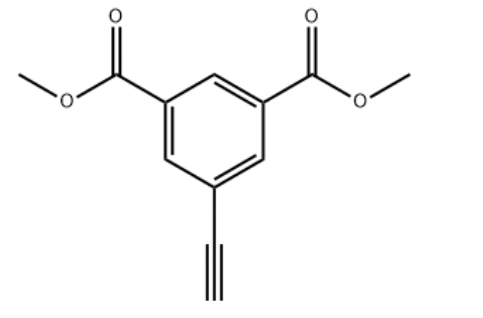
Dimethyl 5-ethynylisophthalate CAS#: 313648-56-5; ChemWhat Code: 1491392
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2019/117733 | THE USE OF ACETYLENE DERIVATIVES IN RUMINANTS | 2019 |
Physical Data
| Melting Point, °C | Solvent (Melting Point) |
| 127 – 128 | |
| 134 – 136 | ethanol |
| 127 – 128 | |
| 124 – 125 | |
| 130 – 131 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 24.84 | 400 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 24.84 | 101 |
| DEPT (Distorsionless Enhancement by Polarisation Transfer), Chemical shifts, Spectrum | 13C | chloroform-d1 | 24.84 | 100 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 24.84 | 100 |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 500 | |
| Chemical shifts | 13C | chloroform-d1 | 125 | |
| Chemical shifts | 1H | chloroform-d1 | 500 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | polyethylene |
| Bands | nujol |
Route of Synthesis (ROS)
| Conditions | Yield |
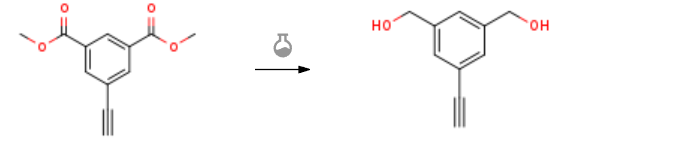
| With lithium aluminium hydride In tetrahydrofuran at 20℃; Experimental Procedure 1.5-1 Step 5-1: Synthesis of e1: In a 250mL flask, weigh e0 (5.00g) and dissolve it in 100mL of THF, add LiAlH4 (2.0eq) under an ice bath, and then warm to room temperature to react overnight;The progress of the reaction is monitored by the dot plate, and the reaction is completely post-processed;The reaction solution was poured into 300 mL of ice water, stirred, suction filtered, washed with water, dried, and dried to obtain a bright yellow solid (3.2 g, 87.7% yield). The hydrogen spectrum of the compound is shown in Figure 8. | 87.7% |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Other Data
| Transportation | Under the room temperature and away from light |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 218.209 |
| logP | 2.392 |
| HBA | 4 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 52.6 |
| Rotatable Bond (RotB) | 4 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Ligand used in a synthesis of heterocycles by palladium-catalyzed C-N cross coupling of 3-bromothiophenes with 2-aminopyridines. Also used in a ruthenium-catalyzed alkylation of active methylene compounds with alcohols. Metal chelating ligand for catalysis. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |