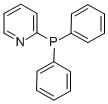
Diphenyl-2-pyridylphosphine CAS#: 37943-90-1; ChemWhat Code: 38623
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2024/79595 | NEW BENZAMIDE DERIVATIVES AS PPAR-GAMMA MODULATORS | 2024 |
| CN113698431 | CHROMENOPYRIDINE DERIVATIVES AS PHOSPHATIDYLINOSITOL PHOSPHATE KINASE INHIBITORS | 2021 |
| KR101920902 | A PET contrast compound for early diagnosis of cardiovascular diseases and use thereof | 2018 |
| US2013/274483 | ASYMMETRIC SYNTHESES FOR SPIRO-OXINDOLE COMPOUNDS USEFUL AS THERAPEUTIC AGENTS | 2013 |
| US2012/184738 | COPPER COMPLEXES FOR OPTOELECTRONIC APPLICATIONS | 2012 |
| WO2011/111806 | OD FOR PRODUCING α, β-UNSATURATED CARBOXYLATE, AND CATALYST FOR PRODUCING THEREOF | 2011 |
| US5326875 | Alkylation of azaglycine derivatives | 1994 |
Physical Data
| Appearance | White to Off-white crystalline powder |
| Melting Point, °C | Solvent (Melting Point) |
| 84.2 – 85.5 | |
| 84.2 – 85.5 | dichloromethane |
| 85 | |
| 82 – 83 | |
| 85 | |
| 84 – 85 | aq. methanol |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 163 | 0.05 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Partner (Association (MCS)) |
| NMR spectrum of the complex | CD2Cl2 | BF4 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | |
| chloroform-d1 | 31P | chloroform-d1 | |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | |
| Chemical shifts, Spectrum | 31P | chloroform-d1 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 600 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 151 |
| Chemical shifts, Spectrum | 31P | chloroform-d1 | 243 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands, Spectrum | |
| ATR (attenuated total reflectance), Spectrum | |
| ATR (attenuated total reflectance), Bands | potassium bromide |
| Bands | neat (no solvent, solid phase) |
| Bands |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | dichloromethane | ||
| Spectrum | |||
| Spectrum | dichloromethane | 258 | 9753 |
| Band assignment, Spectrum | dichloromethane | 276 | |
| Spectrum | dichloromethane | 231, 261, 293 | 8600, 6500, 1100 |
Route of Synthesis (ROS)
| Conditions | Yield |
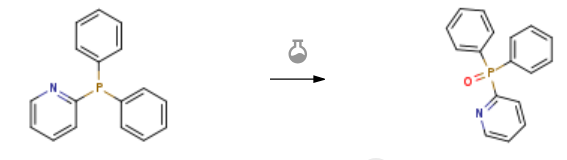
| With dihydrogen peroxide In dichloromethane; water at 20℃; for 3h; Glovebox; Inert atmosphere; | 98% |
| With dihydrogen peroxide In dichloromethane; water at 20℃; for 3h; Glovebox; Inert atmosphere; Experimental Procedure Tris(4-methoxyphenyl)phosphine oxide (2a) General procedure: 1a (70.5 mg, 0.20 mmol), 4-phenylthioxanthone (3 mg, 0.01 mmol), CH3OH (30 mL) were added to a pyrex reaction flash which was equipped with a magnetic stirrer. The mixture was irradiated by a 23 W household lamp at rt under air atmosphere. The photoreaction was completed after 40 minutes as monitored by TLC (eluent: petroleum ether). The solvent was removed and the residue was purified by flash column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 10/1→EA) to afford 2a as a solid (74 mg, 100%); 1H NMR (400 MHz, CDCl3) δ 7.56 (dd, J = 11.6, 8.8 Hz, 6 H), 6.95 (dd, J = 8.8, 2.0 Hz, 6 H), 3.83 (s, 9 H). | 91% |
| With hydrogen In ethyl acetate under 760.051 With dihydrogen peroxide In tetrahydrofuran; water for 0.533333h; | 88% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (97.6%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] H413 (95.1%): May cause long lasting harmful effects to aquatic life [Hazardous to the aquatic environment, long-term hazard] |
| Precautionary Statement Codes | P261, P264, P264+P265, P271, P273, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | |
| Slowly oxidized in the air for a long time, vacuum pack, and store in cold storage | |
| HS Code | |
| Storage | Slowly oxidized in the air for a long time, vacuum pack, and store in cold storage |
| Shelf Life | 1 year |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 263.279 |
| logP | 4.78 |
| HBA | 1 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 26.48 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Ligand in metal-catalyzed reactions: Carbonylation: Facilitates the introduction of carbonyl groups into organic molecules. Hydration: Aids in the addition of water to unsaturated compounds, forming alcohols or other functionalized derivatives. Dehydrogenative coupling: Promotes coupling reactions by removing hydrogen, often forming C-C or C-N bonds. Carbostannylation: Catalyzes the introduction of stannyl (Sn) groups into organic compounds. Dimethylstannylation: Specific variant of stannylation, introducing dimethylstannyl groups. Silylation: Facilitates the introduction of silyl groups, improving stability and reactivity of substrates. Reagent in Mitsunobu reactions: Used in the conversion of alcohols to various functional groups, such as esters, ethers, or amines, under mild conditions. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | https://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |