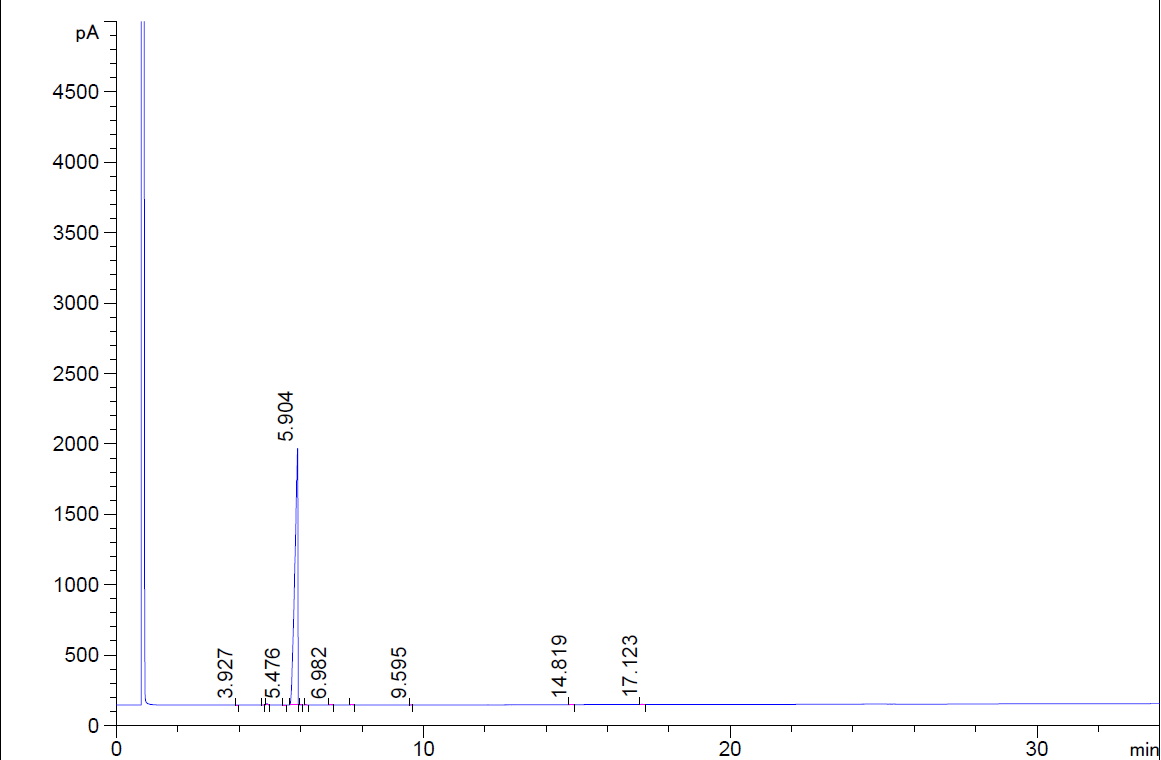
DL-CAMPHORQUINONE CAS#: 10373-78-1; ChemWhat Code: 66295
Identification
| Product Name | DL-CAMPHORQUINONE |
| IUPAC Name | 1,7,7-trimethylbicyclo[2.2.1]heptane-2,3-dione |
| Molecular Structure | 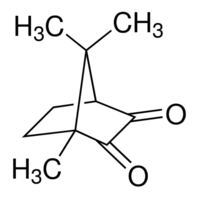 |
| CAS Registry Number | 10373-78-1 |
| EINECS Number | 233-814-1 |
| MDL Number | MFCD00064160 |
| Beilstein Registry Number | 1909463 |
| Synonyms | Camphorquinone |
| Molecular Formula | C10H14O2 |
| Molecular Weight | 166.220 |
| InChI | InChI=1S/C10H14O2/c1-9(2)6-4-5-10(9,3)8(12)7(6)11/h6H,4-5H2,1-3H3 |
| InChI Key | VNQXSTWCDUXYEZ-UHFFFAOYSA-N |
| Canonical SMILES | CC12CCC(C(=O)C1=O)C2(C)C |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2024/41795 | Cyclic Ketone Compounds and Applications Thereof | 2024 |
| US2023/190682 | PHARMACEUTICAL COMPOSITION FOR PREVENTING OR TREATING METABOLIC DISEASES | 2023 |
| US2015/112244 | SELECTIVELY POLYMERIZABLE COMPOSITIONS AND METHODS OF USE IN VIVO | 2015 |
Physical Data
| Appearance | Yellow powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C | Solvent (Melting Point) |
| 201.4 – 202.6 | hexane |
| 198.1 – 199.3 | |
| 190 – 193 | |
| 198 – 200 |
| Boiling Point, °C |
| 251 |
| 250 – 252 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Further physical properties of the complex | H2O | 21.85 | alpha cyclodextrin |
| Further physical properties of the complex | H2O | 21.85 | β-cyclodextrin |
| Association with compound | acetonitrile | ||
| Spectrum of the complex | CDCl3 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 500 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | |
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Chemical shifts | 1H | CCl4 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | potassium bromide | |
| Bands | KBr | |
| Bands | CCl4 | 1780 – 1760 cm**(-1) |
| IR |
| Description (Mass Spectrometry) |
| gas chromatography mass spectrometry (GCMS), electron impact (EI), spectrum |
| EI (Electron impact), Spectrum |
| spectrum, electron impact (EI) |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | acetonitrile | ||
| Spectrum | toluene | 472 | 38 |
| Spectrum | acetonitrile | 466 | 38 |
| Spectrum | water |
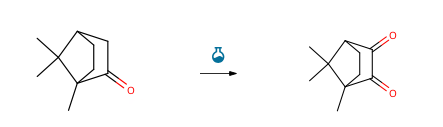
Route of Synthesis (ROS)
| Conditions | Yield |
| With selenium(IV) oxide In ethanol at 150℃; for 1.25h; microwave irradiation; | 92% |
| With manganese(IV) oxide for 0.0222222h; Time; Microwave irradiation; | 87.91% |
| With selenium(IV) oxide; acetic acid In 1,4-dioxane at 78℃; for 12h; Riley Selenium Dioxide Oxidation; | 85% |
| With selenium(IV) oxide; acetic anhydride at 140 – 150℃; for 4h; | 5.22 g |
| Multi-step reaction with 2 steps 1: acetic acid; bromine 2: sodium iodide; cobalt(II) acetate / dimethyl sulfoxide |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (88.9%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (88.9%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (88.9%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 166.22 |
| logP | 1.205 |
| HBA | 2 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 34.14 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Camphorquinone (CAS 10373-78-1) is primarily used as a visible light photoinitiator, especially in dental restorative materials such as composite resins, adhesives, and sealants. It absorbs blue light around 468 nm and, often in combination with amine co-initiators, efficiently generates free radicals to initiate polymerization of (meth)acrylate monomers. Its strong wavelength match with common dental curing lights makes it the standard photoinitiator in dental applications, while it is also applied in certain light-curable resins and adhesives beyond dentistry. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |