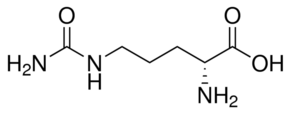
H-D-CIT-OH CAS#: 13594-51-9; ChemWhat Code: 85596
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US6184208 | Peptide, a method for its preparation and a pharmaceutical composition containing the peptide | 2001 |
| EP1323730 | POLYPEPTIDE ANTI-HIV AGENT CONTAINING THE SAME | 2003 |
| US6248716 | Peptide, a method for its preparation and a pharmaceutical composition containing the peptide | 2001 |
Physical Data
| Appearance | White powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C | Solvent (Melting Point) |
| 215 – 218 | Decomposition |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Further physical properties of the complex | H2O | 25 | Citrulline |
| Description (Mechanical & Physical Properties (MCS)) | Solvent (Mechanical & Physical Properties (MCS)) | Temperature (Mechanical & Physical Properties (MCS)), °C | Partner (Mechanical & Physical Properties (MCS)) |
| Virial coefficients | H2O | 24.85 | L-Tryptophan |
| Type (Optical Rotatory Power) | Concentration (Optical Rotatory Power) | Solvent (Optical Rotatory Power) | Optical Rotatory Power, deg | Wavelength (Optical Rotatory Power), nm | Temperature (Optical Rotatory Power), °C |
| [alpha] | 2 g/100ml | aq. HCl | 22 | 589 | 20 |
| [alpha] | 2 g/100ml | aq. HCl | -21.3 | 589 | 20 |
| [alpha] | c=2 | aq. HCl (1n) | -24.2 | 589 | 27 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) |
| Chemical shifts | 1H | D2O |
Route of Synthesis (ROS)
| Conditions | Yield |
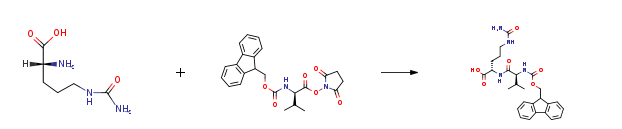
| With sodium hydrogencarbonate In 1,2-dimethoxyethane; water at 25 – 30℃; for 48h; Cooling with ice; Experimental Procedure 2 Synthesis of (S)-2-((S)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-3-methylbutanamido)-5-ureidopentanoic acid (90) Step 2 Synthesis of (S)-2-((S)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-3-methylbutanamido)-5-ureidopentanoic acid (90) To a solution of compound 89 (40.14 g, 0.229 mol) and NaHCO3 (19.23 g, 0.229 mol) in water (750 mL) was added the solution of compound 88 (100 g, 0.229 mol) in DME (750 mL) dropwise under ice-bath. During the addition, a white suspension was formed. Additional THF (400 mL) was added to improve the solubility. After addition, the solution was stirred at 25-30° C. for 2 days. To the reaction was added saturated aq. K2CO3 to adjust pH to 8-9, then extracted with EtOAc (500 mL*5). The aqueous layer was adjusted pH to 3-4 with aq. citric acid. A gelatinous material was formed and filtered. The wet cake was dissolved in THF (1.5 L). Methanol was added until the solid was dissolved. The solution was concentrated in vacuum to remove 30% of solvent and then cooled to room temperature. TBME (2 L) was added to the solution and the mixture was stirred at room temperature overnight. The mixture was filtered and the wet cake was dried in vacuum to give compound 90 as a white solid 60 g (53%). | 53% |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 290621 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 175.188 |
| logP | -3.909 |
| HBA | 6 |
| HBD | 4 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 118.44 |
| Rotatable Bond (RotB) | 6 |
| Matching Veber Rules | 2 |
| Quantitative Results | ||
| 1 of 4 | Comment (Pharmacological Data) | Bioactivities present |
| 2 of 4 | Comment (Pharmacological Data) | Bioactivities present |
| 3 of 4 | Comment (Pharmacological Data) | Bioactivities present |
| 4 of 4 | Comment (Pharmacological Data) | physiological behaviour discussed |
| Use Pattern |
| H-D-CIT-OH CAS#: 13594-51-9 Pharmaceuticals |
| H-D-CIT-OH CAS#: 13594-51-9 an adjuvant |
| vaccine with an enhanced ability to produce an antibody to an influenza virus antigen contained in the vaccine |
| stabilizing agent of composition comprising plasmin or an enzymatically equivalent derivative thereof and matrix metalloproteinase (MMP) inhibitor for effecting or inducing a controlled posterior vitreous detachment (PVD) and treatment of potential complication of a pathological ocular condition |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Apnoke Scientific Ltd | http://www.apnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |