Hexaethylene glycol CAS#: 2615-15-8; ChemWhat Code: 58188
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN108440251 | Photo/nickel synergistic catalysis method for monoarylation of diol | 2018 |
| US2013/209358 | RADIOTRACER COMPOSITIONS | 2013 |
| WO2008/37604 | NOVEL POLYBENZOFULVENE DERIVATIVES, SYNTHESIS AND USES THEREOF | 2008 |
| US2006/230553 | Process for tinting, dyeing or doping of moulded components made of transparent (co)polyamides in aqueous dye bath | 2006 |
| US2003/228275 | DIAZABICYCLOOCTANE DERIVATIVES COMPRISING A QUATERNERY AMMONIUM GROUP FOR USE AS ANTIBACTERIAL AGENTS | 2003 |
Physical Data
| Appearance | Colorless to light yellow clear liquid |
| Melting Point, °C | Pressure (Boiling Point), Torr |
| 197 – 205 | 100 |
| 216 | 1 – 2 |
| 153 – 158 | 0.07 |
| 164 – 168 | 0.05 |
| 217 | 1 |
| 202 – 206 | 0.2 |
| 180 – 189 | 0.3 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.1281 | 4 | 20 |
| 1.127 | 20 | 20 |
| 1.0948 | 4 | 60 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C |
| Chemical shifts | 1H | chloroform-d1 | |
| Chemical shifts | 13C | chloroform-d1 | |
| Chemical shifts | 1H | chloroform-d1 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 25.04 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 25.04 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| ATR-FTIR (attenuated total reflectance Fourier transform infrared spectroscopy), Spectrum | |
| Bands | neat (no solvent) |
| Spectrum | |
| Spectrum | neat (no solvent) |
| Bands | CCl4 |
| Spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Absorption maxima | methanol | 260 |
Route of Synthesis (ROS)
| Conditions | Yield |
| With sodium hydroxide In tetrahydrofuran; water at 0℃; for 5h; Inert atmosphere; Experimental Procedure General procedure for the preparation of tosylate compounds (11a,b) analogously to the description in literature [9] General procedure: A solution of sodium hydroxide (5.48 g, 137 mmol) in water (30 mL) was added to a solution of PEG compound (904 mmol) in THF (30 mL). The resulting mixture was cooled to 0 °C and a solution of p-toluenesulfonyl chloride (16.6 g, 87.4 mmol) in THF (100 mL) was slowly added under stirring for 2 hours. After stirring at 0 °C for 3 hours, the reaction mixture was poured onto an ice/water mixture (500 mL). The organic layer was separated, and the aqueous layer was extracted with dichloromethane (3 × 200 mL). The combined organic layers were washed twice with water (100 mL), dried with MgSO4 and concentrated in vacuo. | 97% |
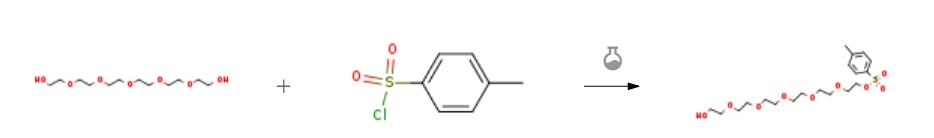
| With potassium iodide; silver(l) oxide at 20℃; for 24h; Inert atmosphere; Experimental Procedure 1 Step 1-2-[2-[2-[2-[2-(2-Hydroxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethyl4-methylbenzene sulfonate To a solution of 2-[2-[2-[2-[2-(2-hydroxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethanol (10.0 g, 35.4 mmol, CAS2615-15-8) in DCM (1.00 L) was added Ag2O (9.85 g, 42.5 mmol), KI (587 mg, 3.54 mmol) and 4-methylbenzenesulfonylchloride (6.75 g, 35.4 mmol). The reaction mixture was stirred under nitrogen atmosphere at rt for 24 h. On completion, the mixture was filtered through a pad of celite and the filtrate was concentrated in vacuo to give a residue. The residue was purified by silica column chromatography (DCM:MeOH=100:1) to give the title compound (15.0 g, 97% yield) as a yellowish oil. 1H NMR (400 MHz, CDCl3) δ 7.73 (d, J=8.4 Hz, 2H), 7.27 (d, J=8.4 Hz, 2H), 4.12-4.06 (m, 2H), 3.68-3.50 (m, 22H), 2.38 (s, 3H). | 97% |
| With potassium iodide; silver(l) oxide In dichloromethane at 20℃; for 18h; | 94% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H319 (23%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] |
| Precautionary Statement Codes | P264+P265, P280, P305+P351+P338, and P337+P317 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under room temperature away from light; keep close and ventilated |
| HS Code | |
| Storage | Under room temperature away from light; keep close and ventilated |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 282.334 |
| logP | -2.158 |
| HBA | 7 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 86.61 |
| Rotatable Bond (RotB) | 16 |
| Matching Veber Rules | 1 |
| Use Pattern |
| Hexaethylene glycol CAS: 2615-15-8 has good solubility and can be used as a polar solvent, particularly in coatings, inks, dyes, and certain polymer materials. It effectively dissolves water and various organic compounds. It is used as a lubricant in high-temperature and extreme environments, especially in some metalworking processes, providing both lubrication and cooling. In the pharmaceutical and cosmetic industries, hexaethylene glycol is a component in lotions, creams, and skincare products, providing moisturizing and skin-softening properties. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Limited | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |