ISOXAZOLE-5-CARBOXYLIC ACID CAS#: 21169-71-1; ChemWhat Code: 225976
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| EP2567958 | Substituted 2-(chroman-6-yloxy)-thiazoles and their use as pharmaceuticals | 2013 |
| US2013/217702 | INDOLE DERIVATIVES | 2013 |
| WO2012/59776 | INDOLE DERIVATIVES | 2012 |
| US2005/171346 | Hexahydropyridazine-3-carboxylic acid derivatives, pharmaceutical compositions containing same and methods of preparation | 2005 |
Physical Data
| Appearance | Light brown crystal or powder |
| Melting Point, °C | Solvent (Melting Point) |
| 138 – 140 | |
| 146 | |
| 145 – 146 | |
| 147 – 148 | benzene, methanol |
| 145.5 – 146.5 | toluene |
| 148 – 149 | toluene |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d1 | 400 | |
| Chemical shifts | 13C | chloroform-d1 | 100 | |
| Chemical shifts | 1H | CDCl3 | 24.85 | 80 |
| Chemical shifts | 13C | CDCl3 | 24.85 | 20 |
| Chemical shifts | 17O | CD3CN | 39.85 | 54.25 |
| Chemical shifts | 1H | CDCl3, dimethylsulfoxide-d6 |
Route of Synthesis (ROS)
| Conditions | Yield |
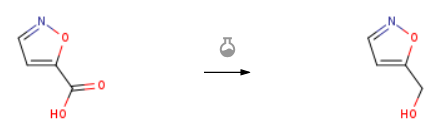
| With borane-THF In tetrahydrofuran at 0 – 20℃; Experimental Procedure To a solution of isoxazole-5-carboxylic acid (1.0 g, 8.8 mmol,) in THF (10 mL) was added borane-THF complex (26.4 mL,26.4 mmol) at 0 °C. The reaction was stirred at room temperature until the substrate was consumed. The reaction was quenched with ethanol (5 mL) at 0 °C. The reaction mixture was partitioned between ethyl acetate and water. The combined organic phase was dried over sodium sulfate, filtered and concentrated to give a crude product which was purified by column chromatography eluting with petroleum ether/ ethyl acetate (2: 1 to give isoxazol-5-ylmethanol (670 mg, 77.0% yield) as a light yellow oil. LCMS retention time 0.329 min; LCMS MH+ 100. | 77% |
| Stage #1: isoxazole-5-carboxylic acid With chloroformic acid ethyl ester; triethylamine In tetrahydrofuran at 0℃; for 0.25h; Stage #2: With sodium tetrahydroborate In tetrahydrofuran; water at 0℃; for 1h; Experimental Procedure Example 55.2-(6-Fluoro- 1 -methyl- 1 H-indazol-3 -yl)-5H-pyrrolo [2,3 -b]pyrazine-7-carboxylic acid (1 – isoxazol-5 – l-ethyl)-amideIn a round-bottomed flask, isoxazole-5-carboxylic acid (1.0 g, 8.84 mmol) was dissolved in THF (35 ml). The solution was cooled to 0°C and triethylamine (1.4 ml, 10.0 mmol) was added followed by ethyl chloroformate (0.94 ml, 9.8 mmol). A thick precipitate was formed upon the addition of the latter. The suspension was stirred at 0°C for 15 min then a solution of sodium borohydride (1.00 g, 26.5 mmol) in water (14 ml) was added portionwise via pipet. Vigorous gas evolution was observed. The reaction mixture was stirred at 0°C for 1 h then diluted with water and saturated aqueous NH4C1 and extracted with dichloromethane (3x). The organic layers were combined, dried over sodium sulfate, filtered and concentrated. The residue waschromato graphed over silica gel with EtOAc/hexanes (gradient 0-50% EtOAc) to afford 513 mg (59%) of isoxazol-5-yl-methanol as a colorless oil. 1H NMR (CDC13, 300 MHz): ? (ppm) 8.23 (d, J=1.9 Hz, 1H), 6.26 – 6.30 (m, 1H), 4.82 (s, 2H), 2.13 (br. s., 1H). | 59% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (90%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under the room temperature and away from light |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 113.073 |
| logP | 0.372 |
| HBA | 2 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 63.33 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |