H-Glu-OtBu CAS#: 45120-30-7; ChemWhat Code: 86232
Identification
Physical Data
| Appearance | White powder |
| Melting Point, °C |
| 147 – 148 |
| 143 – 144 |
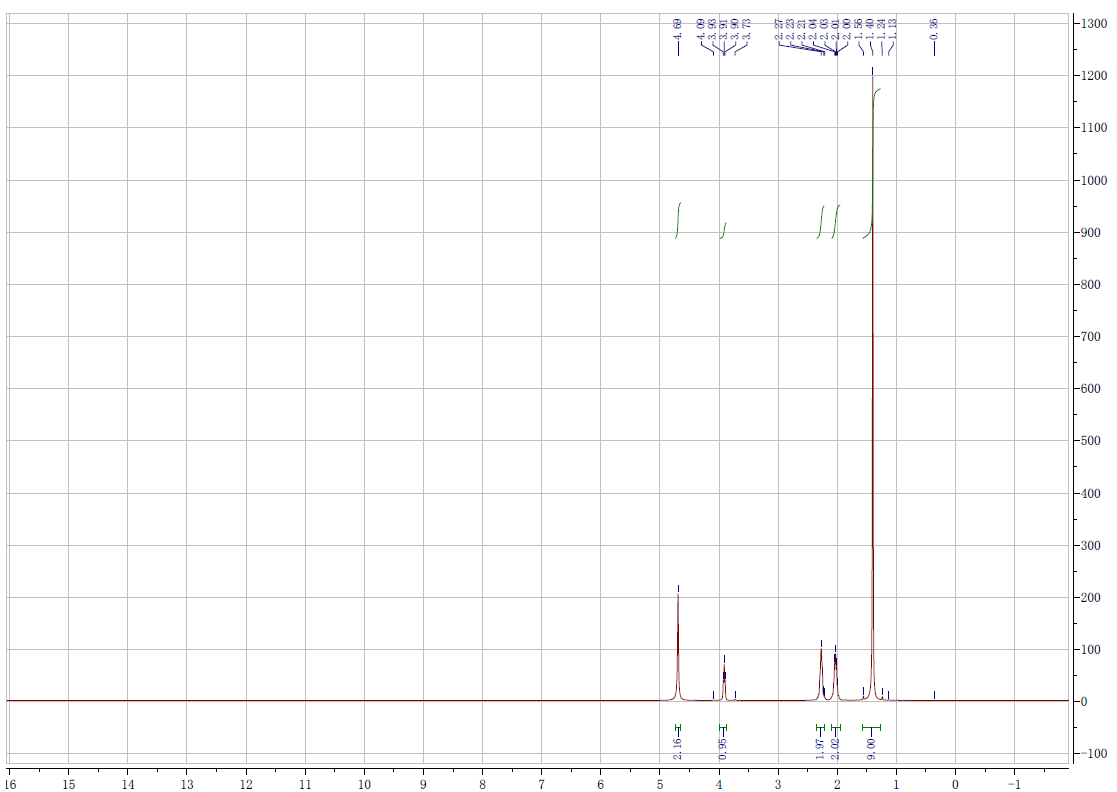
Spectra
Route of Synthesis (ROS)
| Conditions | Yield |
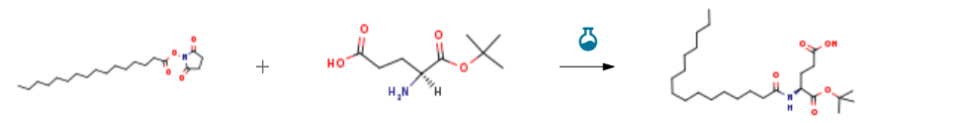
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20 – 30℃; for 3.5h; Experimental Procedure 2 Example 2: Synthesis of Palmitoyl-Glu-OtBu Weigh 160g of Palmitoyl-OSu activated ester (0.45mol),Add 1.6L of dichloromethane,138 g of L-glutamic acid-1-tert-butyl ester (0.0.68 mol) was added, and 87.7 g of N,N’-diisopropylethylamine (0.68 mol) was added dropwise thereto, and the reaction was kept at room temperature (20 ° C to 30 ° C). 3.5 hours.The reaction solution was washed twice with 1.6 L of a 10% aqueous potassium hydrogensulfate solution, and the mixture was separated. The organic phase was collected and washed once with 1.6 L of water. The organic phase was concentrated to dryness.Filtration and drying gave 184 g of Palmitoyl-Glu-OtBu, HPLC purity: 98.6%, yield: 93%. | 93% |
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide for 2h; Experimental Procedure Preparation of N-palmitoyl L-glutamic acid a-t-butoxy ester (ABL3) Preparation of N-palmitoyl L-glutamic acid a-t-butoxy ester (ABL3) Palmitic acid ABL1 (1 .0 g, 3.8 mmol) in THF (10 mL) was treated with N-hydroxy succinimide (0.9 g, 7.6 mmol) and diisopropylcarbodiimide (1 .2 mL, 7.6 mmol) overnight to afford ester (ABL2). The precipitate was removed by filtration, and the volatiles were evaporated in vacuo. The resulting residue was dissolved in DMF (6 mL) and treated with glutamic acid t-butyl ester (0.7 g, 3.4 mmol) and DIEA (1 .8 mL, 10 mmol). After 2 h, the reaction mixture was diluted with water, and the desired product was extracted with Et20. The ether layer was dried over Na2S04, concentrated in vacuo, and the crude mass was purified by Si02 chromatography to afford off-white solid ABL3 (1 .2 g, 74% yield). AP-ESI+ Mass calcd C25H47NO5: 441 .3, Found: 464.0 [M+Na]+ | 74% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Storage | Store at 2~8°, away from light. |
| Shelf Life | 1 year |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 203.238 |
| logP | 0.438 |
| HBA | 5 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 89.62 |
| Rotatable Bond (RotB) | 6 |
| Matching Veber Rules | 2 |
| Use Pattern |
| H-Glu-OtBu CAS#: 45120-30-7 an intermediate in the synthesis of semaglutide and likely plays a crucial role as an intermediate in the synthesis of semaglutide, contributing to its structural integrity, reactivity, and overall efficiency of production. Its inclusion in the synthesis pathway may offer advantages in terms of stability, purity and synthetic efficiency, ultimately supporting the successful development and manufacture of semaglutide for therapeutic use. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Limited | <a href="http://www.caming.com/h-glu-otbu-cas-45120-30-7/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |