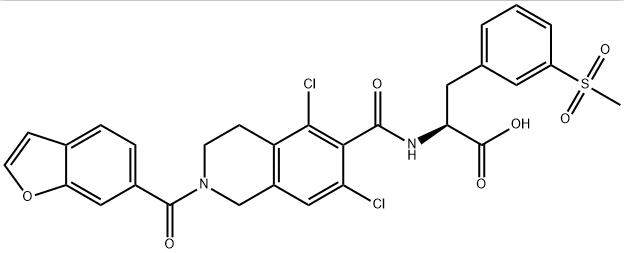
lifitegrast CAS#: 1025967-78-5; ChemWhat Code: 1041101
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2009/54914 | COMPOSITIONS AND METHODS FOR TREATMENT OF DIABETIC RETINOPATHY | 2009 |
Physical Data
| Appearance | White powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C | Solvent (Melting Point) |
| 215 – 220 | |
| 154 – 156 | |
| 154.4 | butanone |
| Type (Optical Rotatory Power) | Concentration (Optical Rotatory Power) | Solvent (Optical Rotatory Power) | Optical Rotatory Power, deg | Wavelength (Optical Rotatory Power), nm | Temperature (Optical Rotatory Power), °C |
| [alpha] | 1 weight percent | methanol | -5 | 589 | 25 |
| Temperature (Solubility (MCS)), °C | Solvent (Solubility (MCS)) | Comment (Solubility (MCS)) |
| 25 | water | Solubility: 90 μg/ml |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz | Original Text (NMR Spectroscopy) |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 300 | ‘HNMR (300MHz, DMSO): d 12.87 (br. s,l H), 9.02 (d, lH,J=8.3 Hz), 8.12 (0100) (d,lH,J=2.2Hz), 7.88 (br. s,lH), 7.79-7.55 (m, 5H), 7.49-7.32 (br. m,2H), 7.05 (dd,lH,J/=2.2 Hz .72=0.9 Hz), 4.80 (br.m,3H), 3.71 (br.s,2H), 3.31 (dd,l H, ;=l4.0 Hz J2=4.5 Hz), 3.16 (s,3H), 3.04 (dd,l H, Ji=\4.0 Hz J2=l0.4 Hz), 2.78 (br.s,2H) ppm. |
| Chemical shifts | 13C | dimethylsulfoxide-d6 | 75 | 13CNMR (75MHZ, DMSO): d 172.5 (s), 169.89 (s), 164.02 (s), 154.12 (s), 148.18 (d), 141.13 (s), 139.56 (s), 137.51 (s), 135.01 (s), 134.91 (d), 132.14 (s), 132.08 (s), 131.62 (s), 129.71 (d), 129.14 (s), 128.87 (s), 128.19 (d), 126.18 (d), 125.51 (d), 122.47 (0102) (d), 121.89 (d), 1 10.78 (d), 107.30 (d), 53.52 (d), 44.70 (t), 44.09 (q), 36.84 (t), 36.32 (t), 26.89 (t) ppm. |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | 300 | |
| Chemical shifts, Spectrum | 13C | dimethylsulfoxide-d6 | 75.5 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands, Spectrum | potassium bromide |
| FT-IR |
| Description (Mass Spectrometry) |
| ESI (Electrospray ionisation), Spectrum |
Route of Synthesis (ROS)
| Conditions | Yield |
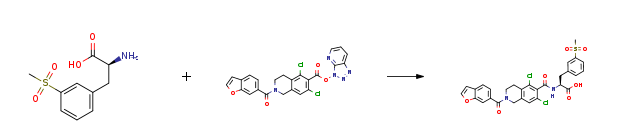
| Stage #1: (S)-2-amino-3-(3-(methylsulfonyl)phenyl)propanoic acid; 3H-[1,2,3]triazolo[4,5-b]pyridin-3-yl 2-(benzofuran-6-carbonyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxylate With N-ethyl-N,N-diisopropylamine In acetonitrile at 40 – 45℃; Stage #2: With hydrogenchloride In water at 0 – 5℃; for 1h; pH=5; | 87% |
| With N-ethyl-N,N-diisopropylamine In water; acetonitrile at 20 – 45℃; for 4h; |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H361 (100%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P201, P202, P281, P308+P313, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | 284200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 615.491 |
| logP | 4.691 |
| HBA | 8 |
| HBD | 2 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 142.37 |
| Rotatable Bond (RotB) | 9 |
| Matching Veber Rules | 1 |
| Use Pattern |
| lifitegrast CAS#: 1025967-78-5 as Pharmaceuticals |
| preparation of a pharmaceutical combination for chronic ocular inflammation |
| treatment of inflammatory eye disorder |
| treatment of dry eye disease |
| lifitegrast CAS#: 1025967-78-5 as dry eye disease |
| immune-related diseases of the ocular surface |
| potent inhibitor of LFA-1 |
| LFA-1 mediated diseases |
| inflammation of the cornea from contact lens wear |
| keratoconjunctivitis sicca (KCS, aka Dry Eye) |
| rejection of corneal transplants Graves’ disease (Basedow disease) |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |