Methoxycarbonyl-L-tert-leucine CAS#: 162537-11-3; ChemWhat Code: 94343
Identification
| Product Name | Methoxycarbonyl-L-tert-leucine |
| IUPAC Name | (2S)-2-(methoxycarbonylamino)-3,3-dimethylbutanoic acid |
| Molecular Structure | 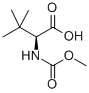 |
| CAS Registry Number | 162537-11-3 |
| MDL Number | MFCD08275827 |
| NACRES | NA.22 |
| Synonyms | (S)-2-(methoxycarbonylamino)-3,3-dimethylbutanoic acid, (S)-2-((methoxycarbonyl)amino)-3,3-dimethylbutanoic acid, (S)-2-(methoxycarbonylamino)-3,3-dimethylbutyric acid, (S)-2-methoxycarbonylamino-3,3-dimethyl-butyric acid, N-(methoxycarbonyl)-L-tert-leucine, N-methoxycarbonyl-(L)-tert-leucine, N-methylxycarbonyl-L-tert-leucine, Ac-Tle-OH, Moc-Tle-OH, 162537-11-3 |
| Molecular Formula | C8H15NO4 |
| Molecular Weight | 189.211 |
| InChI | InChI=1S/C8H15NO4/c1-8(2,3)5(6(10)11)9-7(12)13-4/h5H,1-4H3,(H,9,12)(H,10,11)/t5-/m1/s1 |
| InChI Key | NWPRXAIYBULIEI-RXMQYKEDSA-N |
| Canonical SMILES | CC(C)(C)C(C(=O)O)NC(=O)OC |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| KR2017/31307 | Diphenyl sulfate derivatives or pharmaceutically acceptable salts thereof, preparation method thereof and pharmaceutical composition for use in preventing or treating hepatitis C virus related diseases | 2017 |
| US2014/343290 | PROCESS FOR THE PREPARATION OF ATAZANAVIR OR ITS BISULFATE SALT | 2014 |
| WO2012/122716 | TETRACYCLIC XANTHENE DERIVATIVES AND METHODS OF USE THEREOF FOR TREATMENT OF VIRAL DISEASES | 2012 |
| WO2011/70131 | 5-AMINO- 4-HYDROXYPENTOYL AMIDES | 2011 |
| WO2011/80562 | NOVEL AZA-PEPTIDES CONTAINING 2,2-DISUBSTITUTED CYCLOBUTYL AND/OR SUBSTITUTED ALKOXY BENZYL DERIVATIVES AS ANTIVIRALS | 2011 |
Physical Data
| Appearance | White powder |
| Solubility | Soluble in ethyl acetate and methanol. |
| Melting Point, °C |
| 106-108 |
| 108-109 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz | Original Text (NMR Spectroscopy) |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 300 | 1H NMR (DMSO) δ 12.51 (bs, 1H), 7.28 (d, 1H), 3.80 (d, 1H), 3.53 (s, 3H), 0.93 (s, 9H); |
| Chemical shifts | 1H | chloroform-d1 | 500 | 1H NMR (CDCl3, δ = 7.26 ppm, 500 MHz): 9.57 (br s, 1H), 5.31 (d, 1H), 4.20 (d, 1H), 3.70 (s, 3H), 1.03 (s, 9H) |
| Description (IR Spectroscopy) | Original Text (IR Spectroscopy) |
| in KBr | IR ( Br, cm 1 ): 3379, 2974, 1727, 1688, 1546, 1466, 1332, 1263. 121 1 , 1070, 1034, 1018, 843, 696 |
Route of Synthesis (ROS)
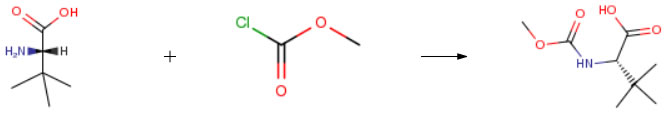
| Conditions | Yield |
| With sodium hydroxide In 1,4-dioxane; water at 60℃; for 18h; pH=8 – 9; Experimental Procedure Step 1: Synthesis of (S)-2-(methoxycarbonylaniino)-3,3-dimethylb tanoic acid:A stirred solution of (S)-2-amino-3,3-dimethylbutanoic acid (about 5.0 g, 38. 16 mmol) in Dioxane (about 20 ml) and sodium hydroxide (2N, 62 ml, PH = 8-9) at about 0 °C, methychlroformate (about 5.88 ml, 76.33 mmol) was added drop wise and stirred at about 60 °C for about 18 hours. The reaction mixture was cooled to room temperature, extracted with DCM, the aqueous layer was separated and acidified with I N HCl. The resulting solution was extracted with EtOAc, dried over Na2S04 and the solvent was evaporated under reduced pressure. The resulting crude was stirred in hexane and decants to afford the title compound as a solid. Wt: 8.5 g: Yield: quantitative; NMR (300 MHZ, CDCI3): δ 5.25 (d, 1 H, J = 10.5 Hz), 4. 19(d, 1 H, J = 9.6 Hz) 3.70 (s, 3H), 1 .03 (s, 9H); Mass: [M- l ]‘ 188 ( 100%); IR ( Br, cm 1 ): 3379, 2974, 1727, 1688, 1546, 1466, 1332, 1263. 121 1 , 1070, 1034, 1018, 843, 696. | 100% |
| With sodium hydroxide In 1,4-dioxane; water at 25 – 60℃; for 22h; Experimental Procedure Example 1; methyl (1S)-1-( (12- [ (2S, 3S)-3-amino-2-hydroxy-4-phenylbutyl]-2- [4- (2- pyridinyl) benzyl] hydrazino} carbonyl)-2, 2-dimethylpropylcarbamate; Example 1A; (2S)-2-[(methoxycarbonyl) amino] -3,3-dimethylbutanoic acid; (L)-tert-Leucine (10 g, 0.076 mol) was dissolved in 1,4-dioxane (40 mL) and treated with 2M NaOH (125 mL, 3.2 equivalents) followed by dropwise addition of methyl chlorofonnate (11.2 mL, 1.9 equivalents) at 25°C. The mixture was heated at 60°C for 22 hrs, cooled, and extracted twice with dichloromethane. The aqueous layer was separated, cooled in ice bath, and acidified with 4N HC1 (60 mL). The mixture was extracted three times with ethyl acetate, and the organic layer was separated, dried with sodium sulfate, filtered, and the solvents were evaporated to give 14.1 g (98%) of the title compound | 98% |
| Stage #1: L-tert-Leucine; methyl chloroformate With sodium hydroxide In 1,4-dioxane; water at 25 – 60℃; for 22h; Stage #2: With hydrogenchloride In water Experimental Procedure 1A (2S)-2-[(methoxycarbonyl)amino]-3,3-dimethylbutanoic Acid EXAMPLE 1A (2S)-2-[(methoxycarbonyl)amino]-3,3-dimethylbutanoic Acid (L)-tert-Leucine (10 g, 0.076 mol) was dissolved in 1,4-dioxane (40 mL) and treated with 2M NaOH (125 mL, 3.2 equivalents) followed by dropwise addition of methyl chloroformate (11.2 mL, 1.9 equivalents) at 25° C. The mixture was heated at 60° C. for 22 hrs, cooled, and extracted twice with dichloromethane. The aqueous layer was separated, cooled in ice bath, and acidified with 4N HCl (60 mL). The mixture was extracted three times with ethyl acetate, and the organic layer was separated, dried with sodium sulfate, filtered, and the solvents were evaporated to give 14.1 g (98%) of the title compound. | 98% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Danger |
| GHS Hazard Statements | H318: Causes serious eye damage [Danger Serious eye damage/eye irritation] H412: Harmful to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P273, P280, P305+P351+P338, P310, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Sealed, keep in a cool, dry place and away from light | |
| HS Code | 294200 |
| Storage | Sealed, keep in a cool, dry place and away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 189.211 |
| logP | 0.839 |
| HBA | 5 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 75.63 |
| Rotatable Bond (RotB) | 5 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Methoxycarbonyl-L-tert-leucine CAS#: 162537-11-3 is an intermediate of API, Atazanavir |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Apnoke Scientific Ltd | http://www.apnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

