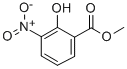
METHYL 2-HYDROXY-3-NITROBENZOATE CAS#: 22621-41-6; ChemWhat Code: 30447
Identification
Physical Data
| Appearance | Sticky yellow crystalline powder |
| Melting Point, °C | Solvent (Melting Point) |
| 129 – 132 | |
| 61 – 63 | |
| 130 – 131 | ethanol |
| 62 | |
| 142 – 143 | |
| 130 – 132 | |
| 128 – 130 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d1 | |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 400 |
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Chemical shifts | 1H | chloroform-d1 | 300 |
| Chemical shifts | 1H | ||
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Chemical shifts | 13C | chloroform-d1 | 100 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| IR | ||
| Spectrum |
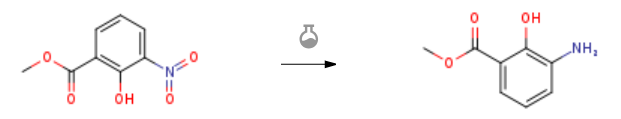
Route of Synthesis (ROS)
| Conditions | Yield |
| With hydrogen; palladium on activated carbon In methanol at 20℃; under 3102.97 Torr; for 49h; Experimental Procedure 68(2) Step 2 Methyl 3-Amino-2-hydroxybenzoate Shake a suspension of methyl 2-hydroxy-3-nitrobenzoate (Step 1,18. 1 g, 93 mmol) and 5% palladium on carbon (5 g) in methanol (200 mL) at room temperature under hydrogen (60 psi) in a Parr bottle for 49 h. Filter the mixture through a plug of Celite and remove the filtrate solvent under reduced pressure to afford methyl 3-amino-2-hydroxybenzoate (Step 2) as a yellow solid (15.6 g, >99%) : 1H NMR (CDC13) 8 10.87 (s, 1H), 7.23 (dd, J = 7.7 Hz, 2H), 6.87 (t, J = 9.9 Hz, 1H), 3.95 (s, 3 H), 3.80 (br s, 2H) ; ESI MS m/z 168 [C8HGNO3 + H] +. | 99% |
| With 10% Pd/C; hydrogen In tetrahydrofuran; ethanol; ethyl acetate at 20℃; Experimental Procedure 51.51-1 Example 51-1; Synthesis of methyl 3-amino-2-hydroxybenzoate Methyl 2-hydroxy-3-nitrobenzoate (1.404 g, 7.12 mmol) in ethanol (70 ml), ethyl acetate (40 ml), was dissolved in THF (40 ml). It was stirred overnight at room temperature under a hydrogen atmosphere with 10% palladium-carbon thereto (140 mg). After the reaction, it was filtered through Celite and evaporated to give the title compound (1.174 g, 98.6%) as a brown solid. | 98.6% |
| With hydrogen; palladium 10% on activated carbon In ethanol at 80℃; under 2625.26 Torr; for 2h; Experimental Procedure To a solution of 2-hydroxy-3-nitro-benzoic acid methyl ester (2.3 g, 11.67 mmol, 1.0 equiv) in ethanol (50 mL) was added palladium on activated charcoal 10% (0.47 g, 0.47 mmol, 0.04 equiv), the reaction vessel filled with hydrogen (3.5 bar) and stirred at 80° C. for 2 h. The catalyst was removed by filtration over celite and the solvent removed under reduced pressure yielding 1.9 g (95%) of the title compound which was used directly without further purification. 1H NMR (300 MHz, CDCl3): δ 3.88 (br s, 2H), 3.93 (s, 3H), 6.71 (t, J=7.9 Hz, 1H), 6.87 (dd, J=7.9 Hz, J=1.5 Hz, 1H), 7.24 (dd, J=7.9 Hz, J=1.5 Hz, 1H). 13C NMR (75 MHz, CDCl3): δ 52.13, 111.83, 118.71, 118.98, 119.55, 135.85, 149.70, 171.11. | 95% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H302 (20%): Harmful if swallowed [Warning Acute toxicity, oral] H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P264, P264+P265, P270, P271, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P319, P321, P330, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under room temperature away from light |
| HS Code | |
| Storage | Under room temperature away from light |
| Shelf Life | 2 years |
| Market Price |
| Use Pattern |
| METHYL 2-HYDROXY-3-NITROBENZOATE CAS#: 22621-41-6 can be used as an intermediate for pesticides, medicines, dyes, chemical fibers, rubber and energetic materials. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |