Methylacetate CAS#: 79-20-9; ChemWhat Code: 1004303
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2023/49476 | CATALYTIC METHODS FOR CARBONYLATION OF ESTERS | 2023 |
| CN116041156 | Synthesis method of 5-(2, 4, 6-trimethylphenyl)-2-propionyl-3-hydroxy-2-cyclohexene-1-ketone | 2023 |
| US2022/81384 | DIRECT CONVERSION OF ESTERS TO CARBOXYLATES | 2022 |
| US2022/380279 | HYDROGENATION OF ESTERS TO ALCOHOLS IN THE PRESENCE OF A RU-PNN COMPLEX | 2022 |
| CN113861058 | Triamidotoluene nucleating agent, and preparation method and use method thereof | 2021 |
Physical Data
| Appearance | Transparent liquid,no visible impurities |
| Methanol | ≤0.01%(m/m) |
| Moisture | ≤0.05%(m/m) |
| Density(20℃) | 0.86(g/cm3) |
| Melting Point, °C |
| -98 |
| -98.15 |
| -98 |
| -98.05 |
| -98.7 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 57 | |
| 56.6 | 751.35 |
| 57 | |
| 56.5 – 57 | |
| 56.25 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 44.99 | |
| 39.99 | |
| 34.99 | |
| 29.99 | |
| 24.99 | |
| 0.90036 | 44.99 |
| 0.90718 | 39.99 |
| Description (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Desorption | nano silica supported 38.3 wt % copper | |
| Sorption table | 20 | poly(phenylene isophthalamide) membrane |
| Sorption table | 20 | poly(phenylene isophthalamide) membrane containing 1 wt % detonation nanodiamond |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C |
| Chemical shifts | 1H | chloroform-d1 | 24.84 |
| Chemical shifts | 13C | chloroform-d1 | 24.84 |
| Chemical shifts, Spectrum | 1H | ||
| Chemical shifts, Spectrum | 1H | benzene-d6 | 24.84 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands, Spectrum | ||
| Intensity of IR bands, Bands, Spectrum | ||
| ATR (attenuated total reflectance), Bands | ||
| Bands, Spectrum | chloroform | |
| ATR (attenuated total reflectance), Bands, Spectrum | diethyl ether | 25 |
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Methylacetate CAS 79-20-9
| Conditions | Yield |
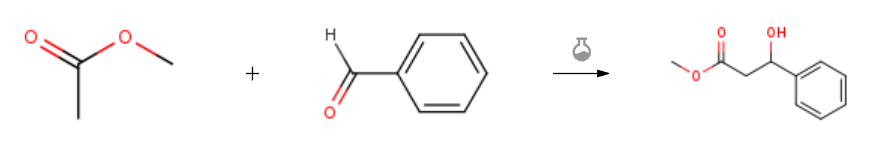
| With n-butyllithium; N,N-diisopropylamine In tetrahydrofuran at -78℃; for 1h; Aldol Condensation; Inert atmosphere; Experimental Procedure General procedure: Under a N2 atmosphere, anhyd i-Pr2NH (870 L, 6.21 mmol) was dissolvedin freshly distilled anhyd THF at 0 °C. The solution was stirredfor 10-15 min and then n-BuLi (3.9 mL, 6.21 mmol) was added dropwise.The mixture was stirred for 15 min at 0 °C and then cooled to-78 °C. This was followed by the dropwise addition of a solution ofmethyl acetate (200 mg, 215 L, 2.7 mmol) in anhyd THF and then themixture was stirred for another 20 min. The freshly distilled aldehyde(3.24 mmol of isobutyraldehyde, 4.05 mmol of acrolein, and 2.7 mmolof benzaldehyde) was then added dropwise. The reaction was monitoredby TLC. At the end of the reaction, sat. aq NH4Cl (20 mL) wasadded and extracted with EtOAc (3 × 15 mL). The combined organiclayers were washed with brine (15 mL), dried (MgSO4) and evaporatedto dryness | 92% |
| With N-ethyl-N,N-diisopropylamine; magnesium(II) iodide In dichloromethane at 20℃; for 0.5h; | 60% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Danger |
| GHS Hazard Statements | H225: Highly Flammable liquid and vapor [Danger Flammable liquids] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H336: May cause drowsiness or dizziness [Warning Specific target organ toxicity, single exposure; Narcotic effects] |
| Precautionary Statement Codes | P210, P233, P240, P241, P242, P243, P261, P264+P265, P271, P280, P303+P361+P353, P304+P340, P305+P351+P338, P319, P337+P317, P370+P378, P403+P233, P403+P235, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
No data available
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 74.0794 |
| logP | 0.241 |
| HBA | 2 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 26.3 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Quantitative Results | ||
| 1 of 386 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Vapor phase carbonylation process using group 4 metal promoted iridium catalyst | |
| 2 of 386 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | OXIME AND AMINE SUBSTITUTED AZABICYCLO AND AZOCYCLO MUSCARINIC AGONISTS AND METHODS OF TREATMENT | |
| 3 of 386 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Optically pure 4-aryl-2-hydroxytetronic acids | |
| 4 of 386 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Nitrogen containing heterobicycles as factor Xa inhibitors | |
| 5 of 386 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | The synthesis and antifungal evaluation of certain acetylenic compounds. | |
| 6 of 386 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | QUINOLINE DERIVATIVES AS PHOSPHODIESTERASE INHIBITORS | |
| 7 of 386 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Tricyclic delta-opioid modulators |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Effect |
| 1 | inhibition rate | 12.5 | % | ||
| 1 | inhibition rate | Not active | |||
| 1 | inhibition rate | Not active | |||
| 1 | inhibition rate | 10 | % | ||
| 1 | inhibition rate | Not active | |||
| 1 | inhibition rate | Not active | |||
| 1 | CC50 (cytotoxic concentration) | > | 1060 | μM | Cytotoxic |
| 1 | CC90 | > | 1060 | μM | Cytotoxic |
| Use Pattern |
| 3-Aminopyridine CAS#: 462-08-8 is an intermediate in pesticides and dyes; pesticide raw materials; analytical reagents. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |