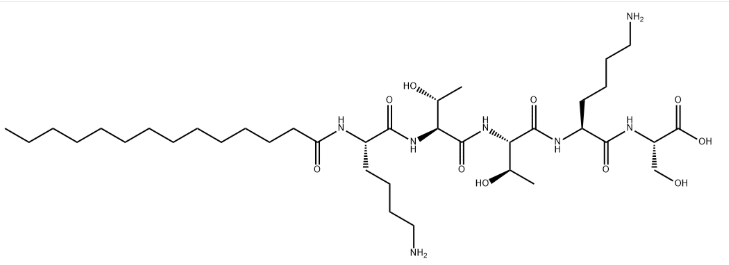
Myristoyl Pentapeptide-4 CAS#: 1392416-25-9; ChemWhat Code: 1491149
Identification
Physical Data
| Appearance | White to off-white powder |
Spectra
No data available
Route of Synthesis (ROS)
| Conditions | Yield |
| Stage #1: With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 24 – 32℃; for 5h; Stage #2: With piperidine In N,N-dimethyl-formamide for 0.25h; Further stages; Experimental Procedure 1 1-1. Resin Swelling General procedure: A solid-phase synthesis reactor equipped with a filtration membrane was used.Peptide synthesis having a carboxyl group (-COOH) at the end of the complex was used with 2-chlorotrityl chloride resin (Bead Tech, Korea),Peptide synthesis was terminated with a peptide bond (-CONH2) at the end of the complex using a link amide resin (GLS, China).Resin was swollen for 20 minutes using dichloromethane and dimethylformamide to prepare the preparation.1-2. Amino acid loadingSynthesis with chlorotrityl chloride resin involves loading the first amino acid into the resin.The resin was removed solvent through a filtration membrane under reduced pressure.3 to 5 equivalents of the amino acid in the resin was completely dissolved in DMF and added to chlorotrityl chloride resin,In consideration of density, diisopropylethylamine was added in an amount corresponding to 3-5 equivalents of chlorotrityl chloride resin.Thereafter, the reaction was performed at 24 ° C. to 32 ° C. for at least 5 hours using a reactor. 1-3. Resin Fmoc DeprotectionSynthesis using the chlorotrityl chloride resin or linkamide resin included a process of deprotection of Fluorenylmethyloxycarbonyl chloride (Fmoc).Resin Fmoc deprotection process removes solvent through the membrane under reduced pressure,After washing for 5 minutes with DMF added 20% (v / v) piperidine, and then again for 10 minutes. The reaction solution was removed by filtration under reduced pressure, and washed six more times for five minutes using DCM or DMF. 1-4. Fatty acid-amino acid complexes and fatty acid- oligopeptide complexes synthesis3 to 5 equivalents of the amino acid in the link amide resin was completely dissolved in DMF, and the solvent was removed to the link amide resin.As a coupling reagent, 2 M HOBt / DIC (hydroxybenzotriazole / diisopropylcarbodiimide) was added to the amino acid equivalent and the link amide resin amount.Then, the synthesis was carried out at 24 ~ 32 5 hours or more using a reactor.After the reaction was completed, the solvent was removed through a filtration membrane under reduced pressure,Washed six times with clean DMF five times.After washing, the amino acids were coupled in sequence in the same manner as the amino acid binding method.Thereafter, 3 equivalents of fatty acid was added to the resin in the state where the peptide was synthesized.2 M HOBt / DIC was added according to amino acid equivalent and resin amount.After using the reactor for 5 hours or more, it was reacted at a temperature of 24 ~ 32 . 1-5. CleavageAfter the reaction was completed, the solvent was removed through the filtration membrane under reduced pressure twice with clean DMF twice for 2 minutes,After washing twice with DCM twice, the solvent was almost removed through the filtration membrane under reduced pressure.The dried peptide complex resin was separated for 4 hours using 70% (v / v) TFA / 29% (v / v) DCM / 1% (v / v) H2O solution.1-6. CrystallizeThe separated solvent was extracted by recrystallization of the crude product using ethyl ether. |
Safety and Hazards
| Precautionary Statement Codes | Not Classified |
Source: European Chemicals Agency (ECHA)
License Note: Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: “Source: European Chemicals Agency, http://echa.europa.eu/”. Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.
License URL: https://echa.europa.eu/web/guest/legal-notice
Record Name: (1-Cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluorophosphate
URL: https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/213446
Description: The information provided here is aggregated from the “Notified classification and labelling” from ECHA’s C&L Inventory. Read more: https://echa.europa.eu/information-on-chemicals/cl-inventory-database
Other Data
| Transportation | Store at -20°C to 8°C for long time, in container tightly sealed; Protect from light and moisture, in dry place |
| HS Code | |
| Storage | Store at -20°C to 8°C for long time, in container tightly sealed; Protect from light and moisture, in dry place |
| Shelf Life | 2 years |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 774.012 |
| logP | 0.03 |
| HBA | 17 |
| HBD | 11 |
| Matching Lipinski Rules | 1 |
| Veber rules component | |
| Polar Surface Area (PSA) | 295.53 |
| Rotatable Bond (RotB) | 38 |
| Matching Veber Rules | 0 |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| Use Pattern |
| Myristoyl Pentapeptide-4 CAS 1392416-25-9 is a fatty acid-modified pentapeptide compound primarily used in cosmetics and skincare products. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Apnoke Scientific Ltd | http://www.apnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |